UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
(Mark One)
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For
the fiscal year ended
Or
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission
file number
(Exact Name of Registrant as Specified in Its Charter)
(State or Other Jurisdiction of Incorporation or Organization) |
(I.R.S. Employer Identification No.) |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s
telephone number, including area code:
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| The
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate
by check if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate
by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate
by check mark if the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act
of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has
been subject to such filing requirements for the past 90 days.
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant
was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |
| ☒ | Smaller reporting company | |||
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate
by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness
of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered
public accounting firm that prepared or issued its audit report.
Indicate
by check mark if the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐
The
aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was $
Indicate the number of shares outstanding of each of the registrant’s classes of common equity, as of December 14, 2022:
| Class | Number of Shares | |
| Common Stock, $.0001 par value |
Documents incorporated by reference
TABLE OF CONTENTS
| -2- |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include all statements that are not historical facts. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential,” or the negative of those terms, and similar expressions and comparable terminology intended to identify forward-looking statements. These statements reflect the Company’s current views with respect to future events and are based on assumptions and subject to risks and uncertainties including those set forth below and under Part I, Item 1A, “Risk Factors” in this annual report on Form 10-K. Given these uncertainties, you should not place undue reliance on these forward-looking statements. These forward-looking statements represent the Company’s estimates and assumptions only as of the date of this annual report on Form 10-K and, except as required by law, the Company undertakes no obligation to update or review publicly any forward-looking statements, whether as a result of new information, future events or otherwise after the date of this annual report on Form 10-K. You should read this annual report on Form 10-K and the documents referenced in this annual report on Form 10-K and filed as exhibits completely and with the understanding that the Company’s actual future results may be materially different from what the Company expects. The Company qualifies all of its forward-looking statements by these cautionary statements. Such statements may include, but are not limited to, statements concerning the following:
● our lack of operating history and history of operating losses;
● our current and future capital requirements and our ability to satisfy our capital needs;
● our ability to complete required clinical trials of our products and obtain approval from the FDA or other regulatory agents in different jurisdictions;
● the potential impact of the recent COVID-19 pandemic on our operations, including on our clinical development plans and timelines;
● our ability to maintain or protect the validity of our patents and other intellectual property;
● our ability to retain key executive members;
● our ability to internally develop new inventions and intellectual property;
● interpretations of current laws and the passages of future laws;
● acceptance of our business model by investors;
● the accuracy of our estimates regarding expenses and capital requirements; and
● our ability to adequately support growth.
| -3- |
PART I
Item 1. Business.
Overview
Sonnet BioTherapeutics Holdings, Inc., is a clinical stage, oncology-focused biotechnology company with a proprietary platform for innovating biologic medicines of single- or bi-specific action. Known as FHAB™ (Fully Human Albumin Binding), the technology utilizes a fully human single chain antibody fragment that binds to and “hitch-hikes” on human serum albumin for transport to target tissues. We designed the FHAB construct to improve drug accumulation in the cancer tumors well as to extend the duration of activity in the body. FHAB development candidates are produced in a mammalian cell culture, which enables glycosylation and a biological structure similar to the natural cytokines in vivo. We believe our FHAB technology, for which we received a U.S. patent in June 2021, is a distinguishing feature of our biopharmaceutical platform that is well suited for future drug development across a range of human disease areas, including oncology, autoimmune, pathogenic, inflammatory, and hematological conditions.
Our current internal pipeline development activities are focused on cytokines, a class of cell signaling peptides that, among other important functions, serve as potent immunomodulatory agents. Working both independently and synergistically, specific cytokines have shown the ability to modulate the activation and maturation of immune cells that fight cancer and pathogens. However, cytokines on their own do not preferentially accumulate in specific tissues and are quickly eliminated from the body, the conventional approach to achieving a treatment effect with cytokine therapy typically requires the administration of high and frequent doses. This can result in a reduced treatment effect accompanied by the potential for systemic toxicity, which poses challenges to the therapeutic application of this class of drugs.
Sonnet has built an efficient R&D platform that includes a network of outsourced vendors to help remediate expenses and improve execution timelines. Most of the vendors are strategic collaborators that offer the company a preferred status with negotiated costs. The major advantages of this approach include optimized direct investment into projects with expenses that can be rapidly scaled up or down depending on the number of projects. The cost advantages of the Sonnet platform start at the vendor network selection process, with CMC being one of the most expensive components of the initial drug development step. Sonnet has chosen a strategic CMC collaborator in India and has negotiated the cost to be significantly less than the expense incurred from a similar US- or Europe-based vendor. Sonnet is conducting two of the company’s three ongoing clinical trials in Australia, which carries a substantial cost reduction relative to US trials via the Australian government’s R&D credit program. Sonnet is also coordinating the Indian and Australian execution with top R&D vendors from the US, England, Germany, and Switzerland, with the objective of directing the bulk of the company’s operating expense infrastructure towards its drug development pipeline.
Pipeline
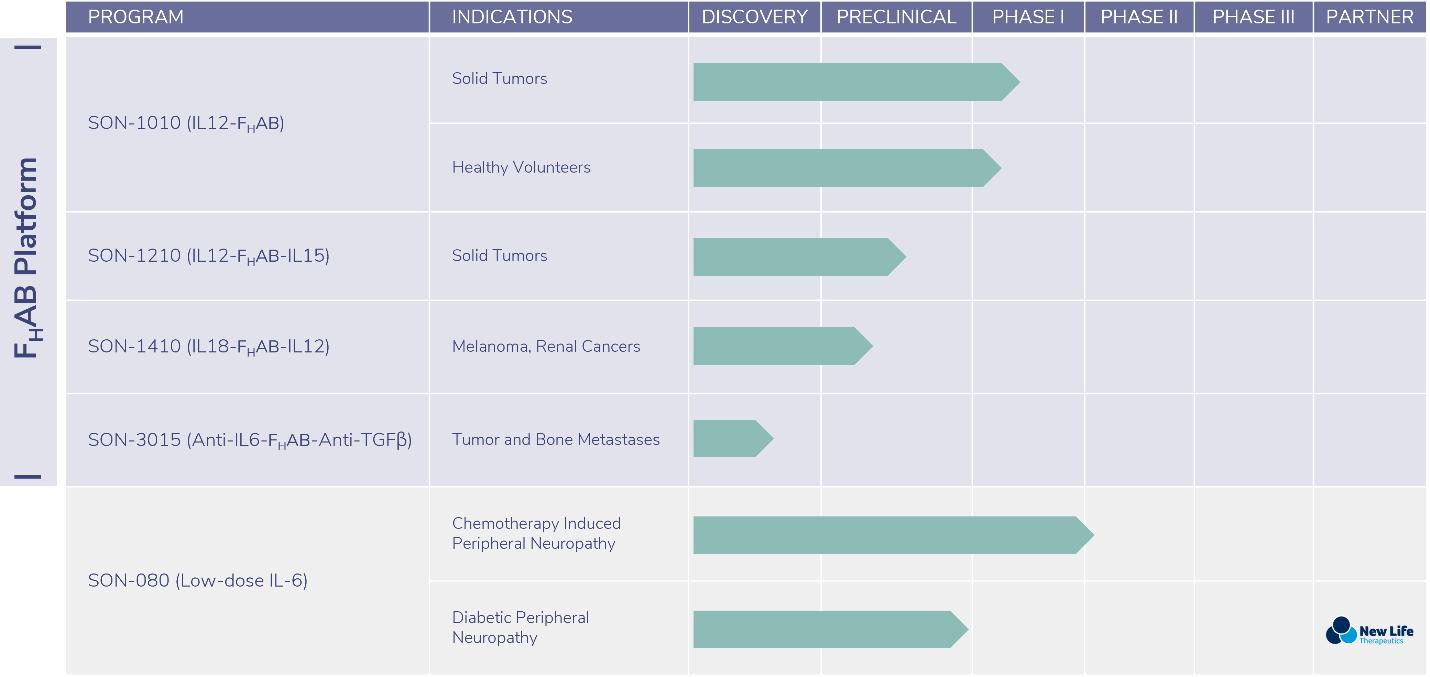
We have a pipeline of therapeutic compounds focused primarily on oncology indications of high unmet medical need.
| ● | Our lead proprietary asset, SON-1010, is a fully human single-chain version of Interleukin 12 (“IL-12”), covalently linked to the FHAB construct, for which we are pursuing clinical development in solid tumors. Sonnet has completed a non-human primate (“NHP”) GLP toxicity study and has successfully manufactured both liquid and lyophilized forms of the drug product for clinical use. During the first half of 2022, we initiated a Phase 1 clinical trial in the US in cancer patients, as well as an Australian clinical trial in healthy volunteers to study the compound’s pharmacokinetics (PK) and pharmacodynamics (PD). The interim safety data from both clinical studies were presented during the fourth calendar quarter of 2022. These data set the stage for the next phase of clinical development, which includes exploring combination treatment with a checkpoint inhibitor. Data from the primary analyses of both trials are expected during the second calendar quarter of 2023. | |
| ● | The Company acquired the global development rights to a fully human version of Interleukin 6 (“IL-6”), in April 2020. We refer to this candidate as SON-080, for its target indications of Chemotherapy-Induced Peripheral Neuropathy (“CIPN”) and Diabetic Peripheral Neuropathy (“DPN”). Sonnet’s CIPN Phase 1B/IIA clinical trial is underway in Australia. This study could yield initial top line clinical safety data during the first half of 2023. Pursuant to a license agreement the Company entered with New Life Therapeutics Pte., Ltd (“New Life”) of Singapore in May 2021, Sonnet and New Life will be jointly responsible for developing SON-080 in DPN with the objective of initiating an ex-US pilot efficacy study in the second half of 2023. | |
| ● | SON-1210 (IL12-FHAB-IL15), our lead bispecific compound, combines the FHAB construct with fully human IL-12 and fully human Interleukin 15 (IL-15). This compound is being developed for solid tumor indications, including colorectal cancer. The in-life portion of a non-human primate (NHP) study is on track to complete in the fourth calendar quarter of 2022, with initiation of the regulatory authorization process to commence during the first half of 2023. Manufacturing and drug supply are secured with a lyophilized drug product formulation being prepared during the first calendar quarter of 2023, which is expected to be suitable for first-in-human clinical trials. |
In our discovery pipeline, we are investigating:
| ● | SON-1410 (IL18-FHAB-IL12), a bispecific combination of Interleukins 18 (IL-18) and 12 (IL-12) for melanoma and renal cancers. Cell line development and process development are ongoing, with early experimental drug supply suitable for formulation and analytical method development activities, in addition to small quantities for use in early development proof-of-concept in vitro studies. Process development activities will continue through 2023, with the potential to generate drug suitable for initial in vivo mouse studies by the end of the 2023 calendar year. |
| ● | SON-3015 (anti-IL6- FHAB-anti-TGFβ), a bispecific combination of anti-IL6 and anti-Tumor Growth Factor Beta for tumor and bone metastases. Early-stage bispecific drug has been generated and has been stored for future use in in vivo mouse studies. Sonnet has elected to place the SON-3015 development program on hold for expense reduction purposes. |
| -4- |
We face numerous challenges and uncertainties with respect to the development and commercialization of our therapeutic compounds, including our FHAB technology. Please see “Risk Factors” contained elsewhere in this prospectus, and the sections entitled “Risk Factors” in the documents incorporated by reference into this prospectus.
Strategy
Our goal is to rapidly advance our pipeline and leverage our therapeutic FHAB platform to become a leader in the discovery, development, and commercialization of biologic drugs. Since its founding, Sonnet has remained focused on rapidly progressing pipeline candidates towards the clinic, while also working to establish collaborations with suitable partners. As partnership conversations evolve, Sonnet intends to prioritize its expense allocation on assets with the greatest strategic interest. To this end, Sonnet is working to reduce operating expenses by approximately 30% and intends to negotiate a licensing deal that will help fund future pipeline expansion. As one example of an announcement in its early stages, Janssen is evaluating three of our pipeline compounds, SON-1010, SON-1210 and SON-1410, in combination with its cell therapy products.
FHAB program advancement: We submitted an IND for SON-1010 to the FDA during the second half of 2021 and completed all of our responses to the agency in early 2022. Our first-in-human clinical study was initiated during the first calendar quarter of 2022. Regarding our first bispecific candidate, SON-1210, GMP bulk drug substance is manufactured and is expected to be ready for clinical applications during the first half of 2023.
Progress SON-080 into the next phase of clinical development: SON-080 is a fully human version of low dose Interleukin 6 (IL-6) being studied for chemotherapy-induced peripheral neuropathy (CIPN). SON-080 has successfully completed Phase I/II clinical trials in cancer patients and we initiated a pilot efficacy Phase Ib/IIa study in CIPN patients during the second half 2022.
Manufacturing platform: Sonnet compounds are produced using an industry standard mammalian cell (Chinese Hamster Ovary/CHO) host cell line that allows for rapid scale-up and commercial manufacturing using state-of-the-art, manufacturing processes and technologies. The mammalian cell culture system enables glycosylation and a similar biological structure to the natural cytokines in vivo. The manufacture of cytokines for clinical applications, namely their production and purification, poses distinct technical challenges. To this end, Sonnet has developed a continuous intensive perfusion process to manufacture commercial yields and we are seeking intellectual property protection for certain of these manufacturing and downstream process development steps.
Regulatory strategy: We believe that Sonnet’s drug candidates are significantly differentiated from existing therapies and represent potential breakthroughs in biopharmaceutical drug development. We will endeavor to seek breakthrough therapy designations with regulatory agencies, which could potentially lead to accelerated clinical development timelines.
Pipeline licensing opportunities: We are pursuing partnering opportunities with leading biopharmaceutical companies for the development and commercialization of our pipeline assets.
FHAB technology expansion: Sonnet is exploring FHAB technology licenses with external partners interested in expanding its therapeutic deployment, which we believe could lead to the platform’s application in other areas, such as vaccines, antibody drug conjugates and as a supplement to chimeric antigen receptor (CAR) T-cell technology. Provisional patents have been filed to secure exclusivity with FHAB in these fields.
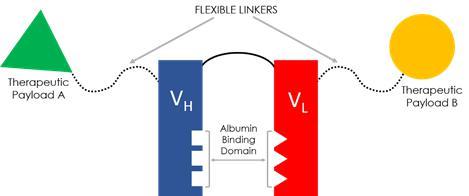
The FHAB Technology
Our proprietary FHAB technology was engineered to address several important shortcomings of existing approaches to biopharmaceutical drug development. We designed FHAB as a plug-and-play, modular construct for innovating new chemical entities that does not need to be reconfigured for different therapeutic payloads. As is the case with all biologic drugs, dose level and frequency of administration are critical variables that oftentimes present as barriers to the development process. After injection, large molecule therapeutics, including peptides, proteins, fusion proteins, antibodies and the like, must remain intact and be capable of reaching their designated targets inside the body, without exceeding specific toxicity thresholds. Finally, they must also be produced using commercially attractive means.
Sonnet’s platform technology was designed to harness human serum albumin (HSA) as a therapeutic shuttling molecule. HSA is naturally present in the bloodstream and the predominant protein in blood plasma. Albumin is a source of energy for inflamed, hypermetabolic tissues, including tumors. Due to the active need for nutrients, cancer cells overexpress albumin-binding proteins such as SPARC (Secreted Protein Acidic and Rich in Cysteine) and gp60 (Albondin glycoprotein).
| -5- |
Pursuant to a Discovery Collaboration Agreement, dated July 23, 2012 and to an Amendment of Discovery Collaboration Agreement, dated May 7, 2019 (together, the “Collaboration Agreement”), XOMA (US) LLC (“XOMA”) granted to Sonnet a non-exclusive, non-transferrable license and/or right to use certain materials, technologies and information related to the discovery, optimization and development of antibodies and related proteins and to develop and commercialize products thereunder (each, a “Product”). The Collaboration Agreement included a license to use a fully human bacteriophage library that was designed to generate fully human single-chain antibody fragments (‘scFv’) comprising a full repertoire of human heavy and light chains for use in panning biological sequences for specific functions. Applying stringent criteria, Sonnet panned millions of scFv binders to HSA to generate Sonnet’s FHAB, which binds to HSA, a globular protein having three major domains. It is known that albumin domains 1 and 3 are involved in the binding to FcRn. This allowed Sonnet to select and characterize scFv binders specific to domain 2, a foundation of Sonnet’s FHAB platform.
Sonnet is obligated to make contingent milestone payments to XOMA totaling $3.75 million on a Product-by-Product basis upon the achievement of certain development and approval milestones related to a Product. Sonnet has also agreed to pay XOMA low single-digit royalties on net sales of Products sold by Sonnet. Royalties on each Product are payable on a country-by-country basis until the later of (i) twelve (12) years after the First Commercial Sale (as defined in the Collaboration Agreement), and (ii) the date of expiration of the last valid claim in the last-to-expire of the issued patents covered by the Collaboration Agreement. In addition, Sonnet has the right to reduce the rate of the royalty on a Product-by-Product basis by paying XOMA a specified amount. The Collaboration Agreement may be terminated by either party for cause and contains customary indemnification provisions.
Sonnet’s FHAB has demonstrated a high binding affinity to serum albumin across species (human, mouse and cynomolgus monkey), with little-to-no immunogenicity, and retains the benefits of neonatal FcRn-mediated recycling of albumin for extending serum half-life by up to four weeks. Unlike monoclonal antibodies (mAbs), this binding occurs without invoking ADCC (antibody-dependent cellular cytotoxicity) and CDC (complement-dependent cytotoxicity). The FHAB construct physically binds serum albumin through an ionic, hydrophobic mechanism, which we believe offers a distinct advantage over technologies that rely on chemical, covalent binding. Once broken, a covalent bond cannot reform, whereas Sonnet’s FHAB is designed with the ability to bind, unbind and rebind to albumin. As albumin seeks albumin receptor gp60 and SPARC, FHAB leverages innate biological mechanisms for targeted delivery of the therapeutic payload to the tumor microenvironment.
Another unique advantage of Sonnet’s FHAB is its linker design. Used for attaching one or two large molecule therapeutic payloads, for single or bispecific activity, our G4S (glycine, serine) peptide linkers are flexible, while being long enough to prevent steric hindrance, and can assume a rod-like configuration for enhanced penetration of tight tissue matrices. In addition to maintaining distance between the therapeutic functional domains, Sonnet linkers are fully human and non-immunogenic across the linker structure, including at the payload binding region. In bispecific constructs, the orientation of the therapeutic payloads can be manipulated to improve potential treatment effects.

| -6- |
As a final key design component, FHAB is produced in mammalian cell culture, specifically Chinese Hamster Ovary (CHO) cells, which enables glycosylation for reducing or potentially eliminating immunogenicity. Using CHO, we have created several different genetic fusion constructs with various low molecular weight therapeutic proteins (e.g., recombinant cytokines such as IL-12, IL-15, IL-18, anti-IL-6 and anti-TGFβ). Recombinant therapeutic proteins, including cytokines, have shown great therapeutic potential, but can lack tissue specificity, which can lead to toxicity. Due to their small (<50kDa) size, cytokines also suffer from a shorter circulation half-life (minutes-to-hours versus 21 days) versus monoclonal antibodies. In mouse and non-human primate models, FHAB-derived compounds have demonstrated substantially greater serum half-lives, improved tissue accumulation and marked tumor reduction activity when compared to their respective naked recombinant cytokines.
In summary, our FHAB technology underpins a modular, versatile scaffold that can be customized to yield a broad array of multi-targeted therapeutic candidates. Relative to existing albumin binding technologies, FHAB is differentiated by possessing a linear, rod-like shape designed for better target tissue penetration, a fully human design to reduce immunogenicity, mammalian glycosylation and FcRn binding for longer serum half-life. Importantly, FHAB-derived therapeutics have the potential for targeted delivery, reduced toxicity and wider therapeutic windows, with the added benefit of utilizing a tailored single or bispecific mechanism of action.
Expanded Applications of the FHAB Technology:
Immunotherapy: We believe that our FHAB platform can innovate biologic drugs that target specific tissues while also increasing therapeutic half-life. As the FHAB construct is designed to enable the simultaneous deployment of two synergistic immunotherapy compounds, we envision a path to previously untapped immunotherapeutic advancements.
Drug Conjugation: With the FHAB technology, various drug compounds can be linked to the FHAB scaffold in combinations that extend beyond our first-wave pipeline of cytokines, which presents opportunities for development across myriad disease areas.
Vaccines: Vaccine developers are seeking to improve vaccine efficiency by conjugating vaccines to natural carriers, such as albumin. We believe the FHAB platform, with its modular scaffold structure, could be an efficient vehicle for delivering vaccines to lymph nodes, improving penetration and presentation, and extending half-life.
CAR T-cell Therapy: CAR T-cell therapy involves genetically modifying a patient’s own T cells to recognize cancer cells for more effectively targeting and killing tumors. We believe targeted Sonnet constructs utilizing interleukins could be systemically co-administered to enhance CAR T-cell efficacy.
Pipeline Overview
The following table summarizes information about pipeline programs where we have disclosed specific target indications:
| -7- |
SON-1010
Interleukin 12 (IL-12) is a circulating cytokine that has been shown to exert multiple effects on innate and adaptive immunity. These immune functions are critical in attacking cancer cells and pathogens. IL-12 is a heterodimeric cytokine produced by dendritic cells, monocytes and macrophages, also known as antigen presenting cells (APC’s). IL-12 has been shown to induce interferon gamma (IFN-ɣ) secretion by T cells and natural killer (NK) cells, promote the expansion and survival of activated T-cells and NK cells, supplement the cytolytic activity of cytotoxic T cells, support the differentiation of Th1 helper effector cells and enhance antibody dependent cellular cytotoxicity (ADCC). IL-12 has also been shown to stimulate in vitro antitumor activity of lymphocytes from patients with cancer and in vivo anti-tumor activity in murine tumor models of melanoma, colon carcinoma, mammary carcinoma and sarcoma.
In May 2021, we announced the successful completion of a GLP repeat-dose study of SON-1010 in non-human primates (NHPs). The objectives of the study were to evaluate the toxicity of SON-1010 in NHP using a subcutaneous, repeat-dose regimen at three different dose levels versus untreated controls and to evaluate the potential reversibility of any adverse findings. Study results included:
| ● | The No Observed Adverse Event Level (NOAEL) following repeated administration was more than 50 times the anticipated equivalent human clinical dose in NHP with no evidence of cytokine release syndrome. | |
| ● | Pharmacokinetic (PK) analysis of serum samples confirmed an enhanced profile of IL12-FHAB over recombinant human IL-12, with a half-life around 40 hours in NHP. | |
| ● | A significant increase in Interferon-γ, a key pleiotropic cytokine associated with anti-tumor mechanisms, was observed following dosing with IL12-FHAB. | |
| ● | SON-1010 related changes in clinical observations, body weight, clinical pathology, cytokines, and immunophenotyping were seen, all of which were consistent with on-target effects previously observed in nonhuman primates. | |
| ● | By Day 38 all study subjects recovered to baseline (pre-study) values. | |
| ● | Repeat dosing administration was tolerated at all dose levels examined. |
In February 2021, we announced the successful completion of an NHP non-GLP repeat-dose toxicology study of SON-1010, the data from which were used to inform the design of the GLP toxicity studies in preparation for IND submission. The objectives of the study were to evaluate the toxicity of SON-1010 in a repeat dose regimen at two different doses and to gather critical data for the design of further IND-enabling safety and toxicity studies. The study included both intravenous and subcutaneous routes of administration with a total of two doses given 14 days apart. The high dosage rate utilized in this study was greater than 50 times the anticipated clinical level of exposure to patients. Study results included:
| ● | Repeat dosing by intravenous and subcutaneous routes of administration was tolerated at both dose levels examined. As is typically observed with IL-12 administration, the white blood cell count dropped, and liver enzymes (ALT and AST) were elevated. These were transient effects that returned to baseline within 7 days following the second dose. | |
| ● | SON-1010-related changes in the physiological observations, body weight, pathology, cytokines and immunophenotyping were seen, all of which were consistent with those on-target effects previously observed in single dose studies. | |
| ● | A significant increase in Interferon-γ levels, a key pleiotropic cytokine associated with anti-tumor activity, was observed following the initial dose of SON-1010 with lower Interferon-γ levels observed following the second dose. This trend follows the published data from other studies of IL-12 in both humans and NHPs. | |
| ● | Pharmacokinetic analysis indicated a mean serum half-life of approximately 40 hours in animals administered SON-1010 via subcutaneous injection. This is consistent with data from the previously conducted dose escalation phase of the study, which demonstrates a substantial improvement in half-life compared to the 13-19-hour half-life of naked, recombinant human IL-12. |
Additionally, SON-1010 has demonstrated, preclinically, a larger reduction of tumor growth compared to IL-12 without FHAB (naked/standalone IL-12) in a mouse model of melanoma. Figure 2 below, from this mouse melanoma study, illustrates SON-1010’s 30-to-50-fold increase in tumor reduction compared to standalone IL-12 WT (wild type).
Furthermore, in the same model, SON-1010 accumulated in tumors in higher concentrations and remained in the serum, spleen, and tumor significantly longer than IL-12 WT without FHAB, potentially enabling less frequent administration and at lower doses.
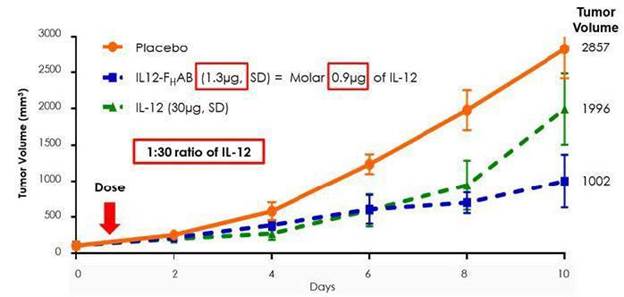
Figure 2: IL-12 (1µg) and IL12- FHAB (1.3µg) are molar equivalent and have similar bioactivity, in vitro; however, in vivo, IL12- FHAB is approximately 30-fold more potent than IL-12 (at day 10, 1.3µg IL12- FHAB > IL-12 30µg).
In another preclinical study using a B16G tumor model, SON-1010 demonstrated an improved dose response versus standalone IL-12 WT, along with increased survival duration (Figures 3 and 4).
Results from this study suggest that SON-1010 may have a greater effect on reducing tumor volume and extending survival versus standalone IL-12 WT.
| -8- |
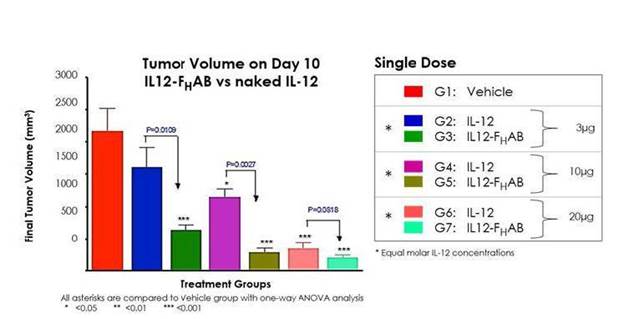
Figure 3: Analysis of tumor volumes shows dose-dependent decreases in tumors in both IL-12 WT and IL12- FHAB-treated mice, as compared to vehicle control. IL12- FHAB-treated mice showed large, statistically significant decreases in tumor volumes when analyzed against equimolar-dosed, IL-12 WT-treated mice. Results suggest IL-12 anti-tumor activity is potentially enhanced with the extension of serum half-life by FHAB linkage.
In Figure 4, below, a Kaplan-Meier analysis was performed to compare survival between animals treated with either SON-1010 or IL-12 WT. These data illustrate a correlation between the percent rate decrease in tumor growth (Figure 3) and an increase in survival duration (Figure 4). In this study, the slower growth of tumors in animals treated with SON-1010 correlates with a longer survival time, as compared to more rapid tumor growth observed with naked IL-12 WT treatment.
Survivability at the lowest doses of SON-1010 (3µg) was equivalent to the highest dose of IL-12 WT (30µg). All doses of SON-1010 showed a 50% survival increase over vehicle at 14 and 17.5 days.
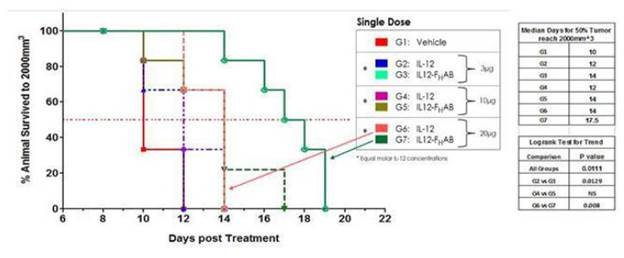
Figure 4: Kaplan-Meier evaluation of mouse B16F tumor survivability shows an increase in survival with IL12- FHAB treatment. Doses of 10µg and 20µg of standalone IL-12 WT exhibited 50% survival at 2 and 4 days over vehicle control (10 days). All doses of IL12- FHAB showed 50% survival over vehicle at 14 and 17.5 days. Survivability at the lowest doses of IL12- FHAB were equivalent to highest dose standalone IL-12.
| -9- |
We have completed in vitro pharmacology studies of affinity and binding kinetics that demonstrate species cross-reactivity of SON-1010 in serum albumin for hamster, rat, cynomolgus monkey and human. The results show that SON-1010 displays species specificity to cynomolgus monkey and human subjects, which will guide species selection for further preclinical toxicology work. A humanized mouse model (SCID) study designed to evaluate PK/PD and dose response is completed. This work informed our decision about dosing in a nonhuman primate (NHP) study.
The objectives of this NHP dose range-finding study were twofold, to confirm the enhanced PK profile of SON-1010 in comparison to recombinant human IL-12 (demonstrated previously in a humanized mouse model), and to perform a dose escalation to inform and de-risk the design of follow-on NHP studies needed for the SON-1010 IND filing with the FDA. The data from this study indicate that in healthy cynomolgus macaques of both sexes, a single dose of SON-1010 is well tolerated at dosage levels greater than 50 times the anticipated exposure in human clinical trials. Additionally, SON-1010 elicited a prolonged and potent on-target PD effect as measured by, Interferon-γ (IFN-γ), a key biomarker of antitumor activity. On-target and transient changes in clinical chemistry and pathology parameters were observed but resolved completely within 14 to 21 days post-dosing. Signs of cytokine imbalance, or uncontrolled increase of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6 were notably absent from all dose levels tested in the study. Pharmacokinetic analysis of serum samples from the study animals indicated a mean half-life of 40.0 (±6.9) hours for the subcutaneous route of administration and 27.45 (±2.8) hours for intravenous dosing. These results build on those from the work with the B16F10 mouse model of melanoma, where the mouse version of SON-1010 showed a 20-fold reduction in the dosage required to achieve a similar therapeutic effect compared to mouse IL-12. Taken together, we believe the observed extended half-life, improved therapeutic window and reduced dosing requirement, made possible by Sonnet’s FHAB technology, represent key advantages of SON-1010 as a potential immune oncology therapeutic.
Work on the master cell bank expressing SON-1010, formulation development and process development activities have all been completed, in addition to drug product formulation (liquid and lyophilized). Process transfer and cGMP product manufacturing is complete. The GLP, IND-enabling toxicology study has been completed. We submitted an IND to FDA during the second half of 2021.
Following FDA approval, we initiated a US clinical trial in oncology patients with solid tumors in April 2022. We also initiated an Australian single-ascending dose (SAD) clinical study in healthy volunteers in July 2022 to carefully study the PK and PD in preparation for potential combination studies. The safety of SON-1010 dosing in several cohorts has been formally reviewed in both Phase 1 clinical trials and dose escalation is continuing as early data becomes available. No dose-limiting toxicities have occurred to date using this novel approach to enhance the safety of the cytokine-based immunotherapy. The clinical dose-escalation strategy was developed based on the ability to use this non-cytotoxic drug in a study in healthy volunteers to rapidly provide clean PK/PD data without interpretation challenges from prior cancer treatment effects. The initial safety and PD data are being used to evaluate the immune response to SON-1010 and predict the effect of further dose escalation. IL-12 has been shown to stimulate the production of interferon-gamma (IFNγ), which is necessary for its antitumor effects. Sonnet is using the known protective effects of IL-12 dose timing to minimize toxicity and extend the maximum tolerated dose (MTD).
SON-080 (Chemotherapy Induced Peripheral Neuropathy)
Through our pipeline expansion efforts, we have identified Interleukin 6 (IL-6) as a cytokine with important biological properties when delivered as a standalone molecule. Our lead clinical stage asset, SON-080, is a fully human version of IL-6 manufactured in Chinese Hamster Ovary (CHO) cells. SON-080 has completed Phase I/II clinical trials in cancer patients with thrombocytopenia and in healthy volunteers and will advance to the next stage of development in chemotherapy-induced peripheral neuropathy (CIPN), a common side effect of treatment with antineoplastic agents in cancer. CIPN is a debilitating condition that manifests itself as pain, numbness and tingling in the extremities. It has been reported in as many as 70% of patients undergoing specific cancer regimens and is a leading cause of patients prematurely aborting chemotherapy. In animal experiments designed to replicate the clinical symptoms of CIPN, SON-080 presented disease-modifying characteristics, including the potential to repair damaged nerves.
Based on the preclinical work, we believe that SON-080 can potentially regenerate damaged nerves, thereby addressing not only the pain-related symptoms, but also the profound discomfort and motor disability CIPN patients often experience. In the nervous system, IL-6 has exhibited potential neurotrophic-like properties, inducing anti-apoptotic gene expression, protecting neurons from toxic injuries, and promoting nerve regeneration and remyelination. SON-080 has demonstrated the potential to elicit nerve regrowth and to re-establish both normal nerve function (Figure 5) and sensations (Figure 6) in various preclinical models of CIPN induced by cisplatin, taxol or vincristine. Activity from treatment with SON-080 was also observed in preclinical models of type 2 diabetic neuropathy and other diseases affecting the nervous system or other organs. This broad activity suggests that the SON-080 mechanism of action might not be restricted to a given class of chemotherapeutic drugs and could elicit a universal neuroprotective-neurorestorative response. Additionally, preclinical data point to the potential of SON-080 to elicit both preventive and curative activity in neuropathies (Figure 6). This introduces the possibility of treating cancer survivors who still suffer from neuropathies, a population representing between 10% and 60% of the 14 million cancer survivors in the US.
| -10- |
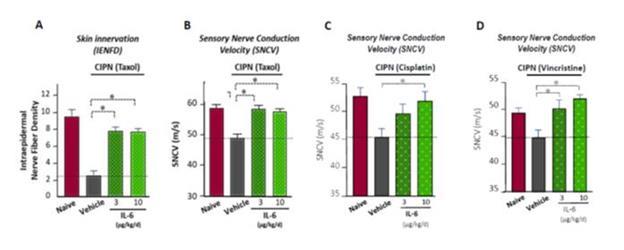
Figure 5: Activity of SON-080 (IL-6) on neuropathy induced by taxol or cisplatin in rats measured at the histological (IENFD) or physiological (SNCV) levels
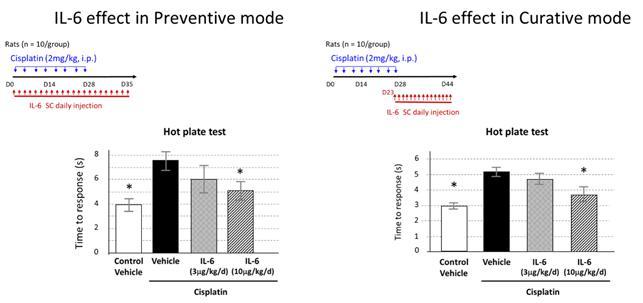
Figure 6: Data show preventive and curative activity potentiating restoration of normal sensitivity (here, using a behavioral response to hot stimulus in cisplatin-induced peripheral neuropathy).
SON-080 has completed Phase I/II studies in over 200 cancer patients with chemotherapy-induced thrombocytopenia. Trial enrollees received subcutaneous doses ranging from 0.25 to 32 µg/kg daily, or thrice weekly. In these trials, where solid tumor cancers were present in more than 75% of the patients treated, the cumulative doses of IL-6 averaged in the 8000 μg range (122 - 54880 μg), and the mean duration of treatment equaled 28 days. One of the trials covered six chemotherapy cycles, with an IL-6 treatment period extending up to 203 days. In none of these trials was an exacerbation of either cancer or neuropathy observed.
The maximum tolerated dose (MTD) of SON-080 was determined in four studies by means of cohort dose escalations of sequential SON-080 dose groups utilizing established common toxicity criteria. When administered daily, the MTD following subcutaneous injection was determined to be between 3 and 8 μg/kg. When given thrice weekly, the MTD was estimated to be > 10 μg/kg. The most clinically relevant apparent toxicities that defined the treatment-limiting dose in these studies were flu-like symptoms and neurocortical toxicity manifested by somnolence, restlessness, confusion, hallucination, and disorientation. We anticipate using a dose that is 50-fold less than the MTD and expect a more benign adverse event profile going forward.
Figure 7 below summarizes the adverse events (AEs) and serious adverse events (SAEs) reported from the Phase I/II clinical studies that are believed to have resulted from treatment with IL-6.
| -11- |
| Total patients (n=214) | No. of AEs in at least 10% of patients treated with IL-6 | No. of SAEs in at least 2% of patients treated with IL-6 | ||||||
| Pyrexia | 151 (70.6%) | 19 (8.9%) | ||||||
| Rigors | 120 (56.1%) | - | ||||||
| Neutropenia | 31 (14.5%) | 15 (7.0%) | ||||||
| Thrombocytopenia | 48 (22.4%) | 15 (7.0%) | ||||||
| Anemia | 64 (29.9%) | 13 (6.1%) | ||||||
| Vomiting | 88 (41.1%) | 10 (4.7%) | ||||||
| Nausea | 106 (49.5%) | 8 (3.7%) | ||||||
| Fatigue | 82 (38.3%) | - | ||||||
| Dehydration | 7 (3.3%) | |||||||
| Dyspnoea | 37 (17.3%) | 7 (3.3%) | ||||||
| Abdominal pain | 27 (12.6%) | 6 (2.8%) | ||||||
| Dizziness | 41 (19.2%) | 5 (2.3%) | ||||||
| Headache | 68 (31.8%) | 5 (2.3%) | ||||||
| Constipation | 51 (23.8%) | - | ||||||
| Diarrhea | 50 (23.4%) | - | ||||||
| Injection site erythema | 46 (21.5%) | - | ||||||
| Fibrinogen increase | 45 (21.0%) | - | ||||||
| Anorexia | 45 (21.0%) | - | ||||||
| Hyperhidrosis | 41 (19.2%) | - | ||||||
| Malaise | 40 (18.7%) | - | ||||||
| Cough | 39 (18.2%) | - | ||||||
| Insomnia | 35 (16.4%) | - | ||||||
| Asthenia | 34 (15.9%) | - | ||||||
| Blood alkaline phosphatase increase | 33 (15.4%) | - | ||||||
| Flu-like symptoms | 28 (13.1%) | - | ||||||
| Alopecia | 28 (13.1%) | - | ||||||
| Mucosal inflammation | 27 (12.6%) | - | ||||||
| Back pain | 26 (12.1%) | - | ||||||
| Lethargy | 26 (12.1%) | - | ||||||
| Pain | 24 (11.2%) | - | ||||||
| Appetite decrease | 24 (11.2%) | - | ||||||
| Bilirubin increase | 23 (10.7%) | - | ||||||
| Arthralgia | 23 (10.7%) | - | ||||||
| Peripheral edema | 22 (10.3%) | - | ||||||
| Platelet count decrease | 22 (10.3%) | - | ||||||
| Hematuria | 22 (10.3%) | - | ||||||
| Veno-occlusive liver disease | - | 5 (2.3%) |
Figure 7: Summary of AEs and SAEs in cancer patients who received IL-6 either concomitantly or following chemotherapy. Doses tested included a range from 0.25 to 26 µg/kg, for a total drug exposure that ranged from 1 to 54,880 mg.
These data form the basis of our forthcoming clinical trials in CIPN, where dosing is expected to be significantly below MTD, as supported by our preclinical studies. For comparison, our target dose will provide a cumulative dose that is 25 times below the mean cumulative dose reached for similar period dosing. We also believe SON-080 has significant potential for treating other neuropathies including diabetic peripheral neuropathy (DPN), as well as potentially other diseases of the nervous system, and we are currently evaluating forward development paths for these opportunities. Sonnet initiated an ex-US Phase 1b/2a pilot-scale efficacy study with SON-080 in CIPN in July 2022. This study could yield initial top line clinical safety data during the first half of 2023.
| -12- |
SON-080 (Diabetic Peripheral Neuropathy)
In addition to our CIPN program with SON-080, our DPN program may, subject to data collected from our planned CIPN studies with SON-080, explore the clinical utility of IL-6 in diabetic peripheral neuropathy (DPN). DPN is currently diagnosed in 50%-80% of the diabetic patient population. According to World Health Organization (WHO) projections, the prevalence of diabetes is estimated to exceed 350 million people in 2030. Neuropathy is progressive and develops over the continuum of diabetes. The condition involves intractable pain with no obvious origin, as well as non-pain-related symptoms such as loss of balance, lack of sensation and autonomic dysfunctions, among others. These deficits impair quality of life and lead to a reduction of life expectancy. Diabetic foot ulcers are a major cost associated with diabetic medical care and are also directly linked to the development of DPN.
Notwithstanding the seriousness of the condition, current treatments only address the pain component of DPN, leaving disease progression and non-pain-related symptoms unaddressed. Furthermore, the few drugs currently used to reduce pain (i.e. Cymbalta, Lyrica, cannabinoids, opioids) are only partially efficacious and are associated with major side effects, which typically delays their introduction into a patient’s care. For these reasons, DPN remains a substantial unmet medical need with high commercial market potential.
Exercise has long been recognized by WHO and caregivers as an effective means of treating and potentially preventing diabetes and several pilot studies have provided evidence to support its role in improving DPN. However, a majority of diabetic patients are physically unable to perform exercise. Regular exercise is known to improve diabetes-associated markers (HbA1c, glucose homeostasis), to ameliorate heart rate variability and to stimulate recovery of both nerve function and blood flow. Recent evidence demonstrates that IL-6 is released during exercise and mediates some of the beneficial effects of physical activity. Sonnet has completed preclinical work in animal models of DPN in which exogenous administration of IL-6 exhibited restorative activity in epidermal nerve density, nerve function, blood flow and reactions to painful or disturbing stimuli. In this context, IL-6 may become a future pivotal disease-modifying therapy for the treatment of DPN.
In vitro data on oligodendrocytes or organotypic cultures have shown that IL-6 potentially induces myelin gene expression by Schwann cells or oligodendrocytes (Figure 8).
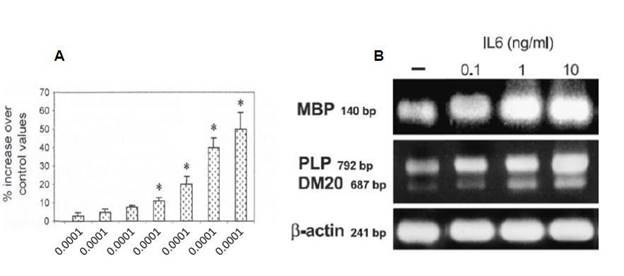
Figure 8: Illustration of survival (A) and differentiation of oligodendrocytes as assessed by myelin basic protein (MBP), proteolipid protein (PLP) and its spliced variant expression (B).
Valerio et al, Mol Cell Neurosci 21 (2002) 602-615.
Pizzi et al, Mol Cell Neurosci 25 (2004) 301-311.
| -13- |
The neuroprotective activity of IL-6 has been evaluated in various paradigms, including excitotoxicity. As well as protecting neurons, IL-6 potentially promotes axonal regeneration and restoration of functional synapses (Figure 9).
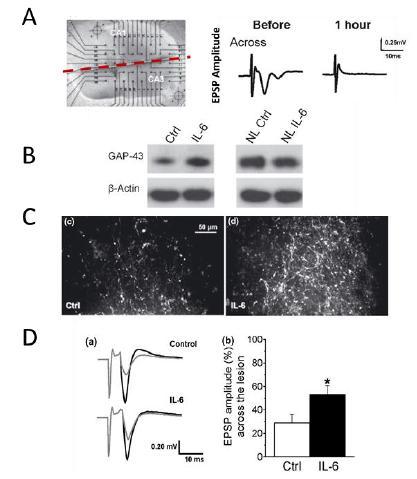
Figure 9: Axonal regeneration activity in hemi-sectioned slices of the hippocampus (A), with increased expression of growth-associated protein 43 (GAP43) in injured slices but not in normal slices (NL) (B). Axonal regeneration activity across the lesion (C) and functional recovery (D) of suppressed (A) excitatory postsynaptic potential (EPSP).
Hakkoum et al, J Neurochem 100 (2007) 747-757.
The activity of IL-6 in preclinical models of DPN has been evaluated by three independent laboratories. This work has shown that IL-6 exhibits positive activity in neuropathy in a dose-dependent manner and may also help restore normal physiological parameters after neuropathy is well established (i.e. four weeks after the induction of diabetes and consequential neuropathy). The beneficial activity is observed on motor (Figure 10-A) and sensory (Figure 10-B) nerve function (conduction velocity), and behaviorally by measuring thermal (Figure 10-C) and tactile (Figure 10-D) perceptions. In addition to the direct effects on myelin and axons previously observed in vitro, IL-6 has also been observed to have activity in restoring microvascular blood flow in the nerve (Figure 10-E), which is a major driver of diabetic neuropathies. Histological analyses of nerves in animals receiving preventive treatment with IL-6 during the development of neuropathy suggest that IL-6 exhibits protective activity on myelin and may play a role in preserving nerve fiber integrity, as well as nerve conduction velocity and the perception of sensations.
| -14- |
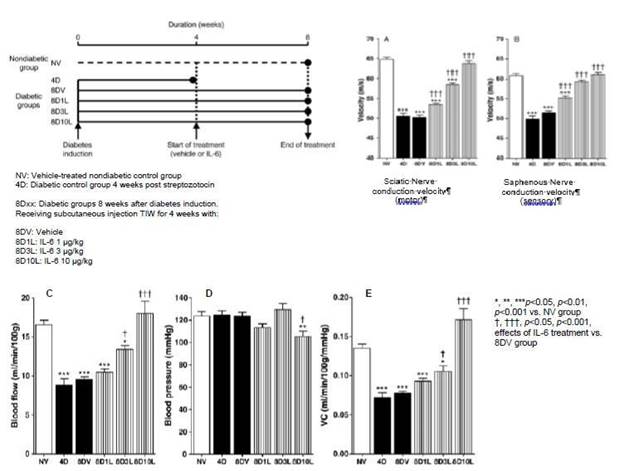
Figure 10: Curative treatment with IL-6 in rats with established diabetic neuropathy induced by streptozotocin.
Cameron et al, Exp Neurol 207 (2007) 23-29.
Outside of oncology, 15 pilot studies totaling 167 subjects, including 27 patients with type 2 diabetes, were conducted by independent academic groups not affiliated with Sonnet to evaluate the role of IL-6 in exercise and metabolism. The peer-reviewed results suggest that low dose IL-6 mimics several beneficial aspects of exercise, including expression of anti-inflammatory molecules, increased lipid metabolism, decreased insulin secretion and activation of the STAT3 signaling pathway in muscle.
We believe these data provide strong support for the clinical development of IL-6 in DPN. Through its mechanism of action and potential disease modifying activity, low dose IL-6 may offer a therapeutic solution for neuropathic symptoms, as well as for cardiac autonomic neuropathies (CAN), in diabetic patients. We intend to use data collected from our CIPN studies with SON-080 to inform our decision about potential next development steps for SON-080 in DPN. Pursuant to the license agreement the Company entered with New Life Therapeutics Pte., Ltd of Singapore in May 2021, we and New Life will be jointly responsible for developing SON-080 in DPN with the objective of initiating an ex-US pilot efficacy study in the second half of 2023.
SON-080: New Life Therapeutics Agreement
In May 2021, we announced the execution of a license agreement, described in detail below (the “New Life Agreement”) which resulted in the out-license of our IL-6 (SON-080) asset for DPN to New Life Therapeutics Pte., Ltd (“New Life”) of Singapore. The licensed territory includes the 10 ASEAN countries of Singapore, Malaysia, Indonesia, Thailand, The Philippines, Cambodia, Brunei, Vietnam, Myanmar and Lao PDR. In June and July of 2021, we amended the New Life Agreement to make Sonnet BioTherapeutics CH SA (rather than Sonnet BioTherapeutics, Inc.) the party to the New Life Agreement (First Amendment) and we also made Sonnet BioTherapeutics, Inc. the Guarantor of performance under the New Life Agreement (Second Amendment), respectively. In addition to the initial $500,000 received by Sonnet upon signing of the LOI in August 2020, an additional $500,000 non-refundable upfront payment was received by Sonnet upon execution of the New Life Agreement. According to the terms of the New Life Agreement, Sonnet could receive a $1 million deferred license fee within 30 days of the achievement of an early commercial sales milestone, a total of up to $19 million in milestone payments and a tiered royalty ranging from 12% to 30% on commercial sales.
Sonnet and New Life intend to initiate an ex-US pilot-scale efficacy study in DPN in the second half of 2023.
| -15- |
SON-1210
SON-1210, our lead bispecific construct, combines IL-12 and IL-15 conjugated to FHAB. These cytokines were selected based on synergistic biologic activity.
IL-15 acts through its specific receptor, IL15Rα, which is expressed on antigen-presenting dendritic cells (APC), monocytes and macrophages. In addition to the potential antitumor properties of IL-12 described above, we believe IL-15 can potentially add the following complementary activity:
| ● | Induce differentiation and proliferation of T, B and natural killer (NK) cells | |
| ● | Enhance cytolytic activity of CD8+ T cells | |
| ● | Induce long-lasting CD8+ memory T cells enhancing immune surveillance against cancer for month/years | |
| ● | Stimulates differentiation and immunoglobulin synthesis by B cells | |
| ● | Induce maturation of dendritic cells | |
| ● | Up regulate IL-12b1 receptor expression |
Summary of the reciprocal biologic activity of Interleukins 12 and 15:
| ● | IL12: Increases IL15Rα receptor, IFNɣ, NK/T cells, TH1 (tumor killing) and decreases Treg | |
| ● | IL15: Increases IL12β 1 receptor, NK cells, CD8 memory and decreases apoptosis |
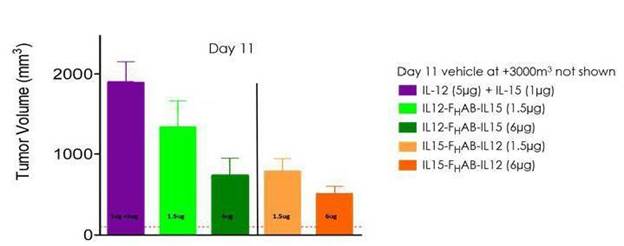
Figure 11: These data suggest an enhanced reduction in tumor growth with SON-1210 compared to concomitantly administered, naked IL-12 and IL-15 in a mouse model of melanoma.
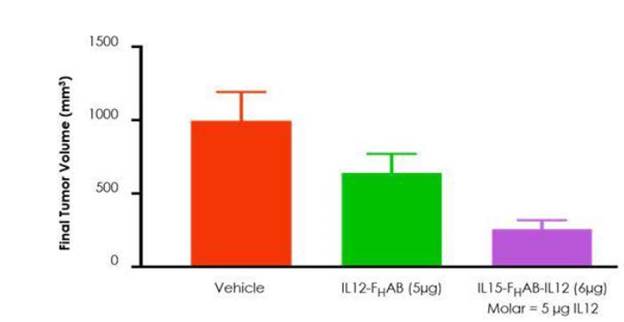
Figure 12: The combination of IL-12 and IL-15 with FHAB displayed synergistic activity, leading to improved tumor volume reduction versus IL12-FHAB alone in a mouse model of melanoma.
A non-human primate (NHP) non-GLP dose escalation study was completed in September 2022, and a GLP repeat dose NHP study has also been completed in the fourth calendar quarter of 2022. cGMP manufacturing for bulk drug is completed, and a lyophilized formulation of drug product will be manufactured in early 2023, to support the First-In-Human (FIH) clinical study. The initial tox material supported the non-GLP study, while the GLP study is being performed on the same lot of GMP drug as intended for the FIH clinical study. The regulatory authorization process for SON-1210 is scheduled to commence during the first half of 2023.
The in-life portion of a non-human primate (NHP) study is on track to be completed in the fourth calendar quarter of 2022, with initiation of the regulatory authorization process to commence during the first half of 2023. Manufacturing and drug supply are secured with a lyophilized drug product formulation being prepared during the first calendar quarter of 2023, which is expected to be suitable for first-in-human clinical trials.
| -16- |
Discovery Assets: SON-1410 (IL18-FHAB-IL12) and SON-3015 (Anti IL6-FHAB-Anti TGFβ)
In August 2021, we announced the selection of a novel development candidate after completing comparative studies in a mouse melanoma model. The candidate represents Sonnet’s second bispecific compound integrating Interleukin 12 (IL-12) with the company’s Fully Human Albumin Binding (FHAB) platform. The target indications for SON-1410 will be melanoma and renal cancers.
IL18-FHAB-IL12 showed statistically significant tumor size reduction in a mouse melanoma study compared with the placebo, as well as a dose response. The data demonstrated:
| Compound | Day 0, Single Dose Tumor @ 100 mm3 | Day 8 Tumor Volume (mm3 +/- SEM), N=8 | Day 8 Percentage Tumor Shrinkage | |||||
| Placebo | NA | 1747 +/- 301 | - | |||||
| IL18-FHAB-IL12 | 1 µg | 918 +/- 130 | 47 | % | ||||
| IL18-FHAB-IL12 | 5 µg | 619 +/- 141 | 65 | % | ||||
A separate mouse study was also performed comparing the selected version of IL18-FHAB-IL12 with two other candidates, GMCSF-FHAB-IL18 and GMCSF-FHAB-IL12. The comparison data indicated significantly greater reduction in tumor volume, along with higher Interferon Gamma levels and immune cell responses (NK, NKT, Th1, and cytotoxic CD8 T cells) using IL18-FHAB-IL12, compared with GMCSF-FHAB-IL12 or GMCSF-FHAB-IL18.
Cell line development activities are being initiated for SON-1410, followed by process development, formulation development and scale-up manufacturing. Development activities for the compound are ongoing, with the potential to generate drug suitable for initial in vivo mouse studies by the end of the 2023 calendar year.
TGF-β1/IL-6 biology is a strong predictor of overall survival in cancer, and the combined targeting of IL-6 and TGF-β1 signaling using SON-3015 may represent a promising strategy for treating tumor and bone metastases. TGFβ is released from degraded bone, and enhances IL-6 production, contributing to the vicious circle of bone metastasis. High FcRn expression in the bone environment would result in accumulation in the bone of the dual construct anti IL6- FHAB-anti TGFβ, thereby potentially inhibiting or blocking bone metastases. Sonnet has elected to place the SON-3015 development program on hold for expense reduction purposes.
| -17- |
We face numerous challenges and uncertainties with respect to the development and commercialization of our therapeutic compounds, including our FHAB technology. Please see “Risk Factors” contained elsewhere in this prospectus, and the sections entitled “Risk Factors” in the documents incorporated by reference into this prospectus.
Competition
The pharmaceutical and biotechnology industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our technology, development experience and scientific knowledge provide us with competitive advantages, we face potential competition from many different sources, including large pharmaceutical and biotechnology companies, academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for the research, development, manufacturing and commercialization of cancer immunotherapies. Any product candidates that we successfully develop and commercialize will compete with new immunotherapies that may become available in the future.
We compete in the segments of the pharmaceutical, biotechnology and other related markets that develop immuno-oncology treatments. There are many other companies that have commercialized and/or are developing immuno-oncology treatments for cancer including large pharmaceutical and biotechnology companies, such as Amgen, AstraZeneca/MedImmune, Bristol-Myers Squibb, Merck, Novartis, Pfizer and Roche/Genentech.
We face significant competition from pharmaceutical and biotechnology companies that target the use of specific cytokines or other large molecules as immunomodulating therapies in the cancer setting. These generally include, single- or bi-specific antibodies, fusion proteins, antibody drug conjugates and targeted vaccines.
With respect to our lead product candidate, SON-080, we are aware of other companies developing products to treat CIPN, including but not limited to Apexian Pharmaceuticals, Inc., Aphios Corporation, Asahi Kasei Corporation, MundiPharma EDO and Regenacy Pharmaceuticals, Inc; however, we believe we are the only company studying the use of a disease-modifying cytokine for the indication.
With respect to our first FHAB-derived candidate, SON-1010, we are aware of other competing IL-12 programs, which include, but are not limited to those being developed by Celsion Corporation, Eli Lilly, Inovio Pharmaceuticals, Inc., Intrexon Corporation, Codiak Biosciences, Xiolio Therapeutics, Werewolf Therapeutics, Dragonfly Therapeutics and OncoSec Medical. We believe that our FHAB integrated IL-12 is tumor-targeted with an enhanced pK profile that differentiates it from the competition.
With respect to our earlier stage pipeline FHAB product candidates SON-1210, SON-2014 and SON-3105, we are not aware of any other competing companies working on these specific bi-specific programs.
Many of the companies against which we are competing or against which we may compete in the future have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved drugs than we do. Mergers and acquisitions in the pharmaceutical, biotechnology and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and enrolling subjects for our clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
We could see a reduction or elimination of our commercial opportunity if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we or our collaborators may develop. Our competitors also may obtain FDA or foreign regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we or our collaborators are able to enter the market. The key competitive factors affecting the success of all our product candidates, if approved, are likely to be their efficacy, safety, convenience, price, the effectiveness of companion diagnostics, if required, the level of biosimilar or generic competition and the availability of reimbursement from government and other third-party payors.
| -18- |
Manufacturing
We rely on contract development and manufacturing organizations, or CDMOs, to produce our drug candidates in accordance with the FDA’s current Good Manufacturing Practices, or cGMP, regulations for use in our clinical trials. The manufacture of biopharmaceuticals is subject to extensive cGMP regulations, which impose various procedural and documentation requirements and govern all areas of record keeping, production processes and controls, personnel and quality control. Our pipeline molecules are manufactured using the standard industrial Chinese Hamster Ovary (CHO) platform with common bio-chemical engineering from readily available raw materials.
To meet our projected needs for clinical supplies to support our activities through regulatory approval and commercial manufacturing, the CDMOs with whom we currently work will need to increase the scale of production, or we will need to secure alternate suppliers. We believe that our current CDMO has the wherewith-all and interest, to add capacity as required. The landscape for CDMOs is strong and there are multiple potential sources for contract manufacturing. We have not yet engaged alternate suppliers in the event that our current CDMOs are unable to scale production, however we have engaged our existing CDMO in expansion conversations. Our relationships with CDMOs are managed by internal personnel with extensive experience in pharmaceutical development and manufacturing.
If we are unable to obtain sufficient quantities of drug candidates or receive raw materials in a timely manner, we could be required to delay our ongoing clinical trials and seek alternative manufacturers, which would be costly and time-consuming.
License and Other Commercial Arrangements
Janssen Pharmaceuticals (Johnson & Johnson)
In October 2022, Sonnet announced a collaboration agreement with Janssen Biotech, Inc. (Janssen), one of the Janssen Pharmaceutical Companies of Johnson & Johnson, where in vitro and in vivo efficacy of SON-1010 (IL12-FHAB), SON-1210 (IL12-FHAB-IL15) and SON-1410 (IL18-FHAB-IL12) will be evaluated in combination with certain Janssen proprietary cell therapy assets. The agreement was facilitated by Johnson & Johnson Innovation. Under the terms of the agreement, Sonnet shall supply the three referenced compounds for use in head-to-head in vitro and in vivo efficacy studies. If successful and subject to provisions of the agreement, Janssen could exercise its option and Sonnet could then seek an expanded collaboration.
New Life
In May 2021, the Company entered into a License Agreement (the “New Life Agreement”) with New Life Therapeutics PTE, LTD (“New Life”). Under the New Life Agreement, the Company granted New Life an exclusive license (with the right to sublicense) to develop and commercialize pharmaceutical preparations containing a specific recombinant human interleukin-6, SON-080 (the “Compound”) (such preparations, the “Products”) for the prevention, treatment or palliation of diabetic peripheral neuropathy in humans (the “DPN Field”) in Malaysia, Singapore, Indonesia, Thailand, Philippines, Vietnam, Brunei, Myanmar, Lao PDR and Cambodia (the “Exclusive Territory”). New Life may exercise an option to expand (1) the field of the exclusive license to include the prevention, treatment or palliation of chemotherapy-induced peripheral neuropathy in humans (the “CIPN Field”), which option is non-exclusive and will expire on December 31, 2021; and/or (2) the territorial scope of the license to include the People’s Republic of China, Hong Kong and/or India, which option is exclusive and will also expire on December 31, 2021. If these options are exercised, the terms of the CIPN Field and the territory expansion will be negotiated by the parties. In June and July of 2021, we amended the New Life Agreement to make Sonnet BioTherapeutics CH SA (rather than Sonnet BioTherapeutics, Inc.) the party to the New Life Agreement (First Amendment) and we also made Sonnet BioTherapeutics, Inc. the Guarantor of performance under the New Life Agreement (Second Amendment), respectively.
The Company will retain all rights to manufacture Compounds and Products anywhere in the world. The Company and New Life shall enter into a follow-on supply agreement pursuant to which the Company shall supply to New Life Products for development and commercialization thereof in the DPN Field (and the CIPN Field, if applicable) in the Exclusive Territory on terms to be negotiated by the parties. The Company will also assist in transferring certain preclinical and clinical development know-how that is instrumental in New Life’s ability to benefit from the license.
| -19- |
New Life will bear the cost of, and be responsible for, among other things, conducting clinical studies and additional non-clinical studies and other developmental and regulatory activities for and commercializing Products in the DPN Field (and the CIPN Field, if applicable) in the Exclusive Territory.
New Life paid the Company a $0.5 million non-refundable upfront cash payment in August 2020 upon executing a letter of intent to negotiate a license agreement and a $0.5 million non-refundable upfront cash payment in June 2021 in connection with the execution of the New Life Agreement. New Life is also obligated to pay a non-refundable deferred license fee of an additional $1.0 million at the time of the satisfaction of certain milestones, as well as potential additional milestone payments to the Company of up to $19.0 million subject to the achievement of certain development and commercialization milestones. In addition, during the Royalty Term (as defined below), New Life is obligated to pay the Company tiered double digit royalties ranging from 12% to 30% based on annual net sales of Products in the Exclusive Territory. The “Royalty Term” means, on a Product-by-Product and a country-by-country basis in the Exclusive Territory, the period commencing on the date of the first commercial sale (subject to certain conditions) of such Product in such country in the Exclusive Territory and continuing until New Life ceases commercialization of such Product in the DPN Field (or CIPN Field, if applicable).
The New Life Agreement will remain in effect on a Product-by-Product, country-by-country basis and will expire upon the expiration of the Royalty Term for the last-to-expire Product in the last-to-expire country, subject to (i) each party’s early termination rights including for material breach or insolvency or bankruptcy of the other party and (ii) the Company’s Buy Back Right and New Life’s Give Back Right (as defined below).
In addition, New Life granted to the Company an exclusive option to buy back the rights granted by the Company to New Life and the Company granted New Life the right to give back the rights with respect to Products in the DPN Field and/or the CIPN Field (if applicable) in one or more countries in the Exclusive Territory on terms to be agreed upon, which options will expire upon the initiation of a Phase III Trial for the applicable Product.
XOMA
Sonnet (as successor-in-interest to Oncobiologics, Inc. (“Oncobiologics”), after Oncobiologics spun-off certain assets into Sonnet and concurrently distributed all of its shares in Sonnet on a pro rata basis to Oncobiologics’s stockholders on April 6, 2015) and XOMA (US) LLC (“XOMA”) are party to a Discovery Collaboration Agreement, dated July 23, 2012 and an Amendment of Discovery Collaboration Agreement, dated May 7, 2019 (together, the “Collaboration Agreement”) pursuant to which XOMA granted to Sonnet a non-exclusive, non-transferrable license and/or right to use certain materials, technologies and related information related to discovery, optimization and development of antibodies and related proteins and to develop and commercialize products thereunder (each, a “Product”). Sonnet is obligated to make contingent milestone payments to XOMA totaling $3.75 million on a Product-by-Product basis upon the achievement of certain development and approval milestones related to a Product. Sonnet has also agreed to pay XOMA low single-digit royalties on net sales of Products sold by Sonnet. Royalties on each Product are payable on a country-by-country basis until the later of (i) a specified period of time after the First Commercial Sale (as defined in the Collaboration Agreement), and (ii) the date of expiration of the last valid claim in the last-to-expire of the issued patents covered by the Collaboration Agreement. In addition, Sonnet has the right to reduce the rate of the royalty on a Product-by-Product basis by paying XOMA a specified amount. The Collaboration Agreement may be terminated by either party for cause and contains customary indemnification provisions.
ARES
On August 28, 2015, Relief, now a wholly owned subsidiary of Sonnet, signed a License Agreement (the “ARES License Agreement”) with Ares Trading, a wholly owned subsidiary of Merck KGaA (“ARES”). Under the terms of the ARES License Agreement, ARES has granted the Company a sublicensable, exclusive, worldwide, royalty-bearing license on proprietary patents to research, develop, use and commercialize products (each, a “Product”) using atexakin alfa (“Atexakin”), a low dose formulation of human interleukin-6 in peripheral neuropathies and vascular complications. Three patents are included in the ARES License Agreement that protect the use of Atexakin to treat i) diabetic neuropathy, ii) chemotherapy-induced peripheral neuropathy and iii) vascular complications.
| -20- |
Pursuant to the ARES License Agreement, we will pay ARES high single-digit royalties on net sales of Products sold by the Company. Royalties are payable on a Product-by-Product and country-by-country basis until the later of (i) a specified period of time after the First Commercial Sale (as defined in the ARES License Agreement) in such country, and (ii) the last date on which such product is covered by a valid claim in such country. If a Product is not covered by a valid claim in a country or such valid claim has expired or been invalidated before the twelfth (12th) anniversary of the date of the First Commercial Sale of such Product in such country, then the royalty rate will be reduced by fifty percent (50%). We will also pay ARES a sublicensing fee that is a percentage of the proceeds received from a sublicensing event (“Sublicensing Receipts”) using a sliding scale (which percentage decreases at later stages of clinical development at which the sublicensing event occurs) that starts in the low double digits and decreases to the high single digits. The ARES License Agreement may be terminated by the Company for convenience at any time or by either party upon a breach by the other party. The License agreement contains customary indemnification provisions.
The Ares License Agreement was amended effective November 1, 2021, in order to clarify the application of some of the terms and conditions contained therein related to sublicensing. In particular:
| ● | Sonnet is now authorized to grant sublicenses to third parties without the prior written consent of ARES, providing that the financial condition of any such sublicenses reflects fair market value as determined by Sonnet in good faith. | |
| ● | Because the initial conditions by which Sonnet would remunerate ARES out of Sublicensing Receipts were unclear, the ARES License Agreement was clarified such that Sonnet will now have to pay ARES a percentage of all Sublicensing Receipts in case the relevant sublicense agreement is signed before or after completion of the first Phase I clinical trial (as opposed to payment only in case the relevant sublicense agreement is signed after completion of the first Phase I clinical trial, as was set in the original ARES License Agreement). | |
| ● | It was agreed that the foregoing clarification would only apply to future sublicensing agreements, and with respect to the royalties (but not the milestone payments) that may be generated from the New Life Agreement. |
Intellectual Property
With respect to our patent portfolio, we have two issued patents (U.S. and Japan), and we have filed patent applications directed to numerous fusion proteins that include the Fully Human Albumin Binding domain (FHAB). If granted, the resulting patents would expire on dates ranging from 2038 to 2041, subject to patent term extensions under certain circumstances. The patent application filings include:
● National filings corresponding to WO/2018/151868 - This application is directed to fully human “Albumin Binding Domain (FHAB) Fusion Proteins”, including fusion proteins with scFv’s (e.g., anti-TGFβ, PD-L1, TNF, IL1, IL6, IL8, etc.), fusion proteins with cytokines (e.g., IL2-FHAB, IL12-FHAB, IL15-FHAB, IL7-FHAB, etc.) and combinations of two cytokines, such as IL12-FHAB-IL15, GMcSF-FHAB-IL18, and IL18-FHAB-IL12; and methods of treatments using such FHAB fusion proteins. A patent was issued in the United States on June 8, 2021, as U.S. Patent No. 11,028,166. Additionally, a Decision to Grant was received in the Japan national stage application. U.S. Patent No. 11,028,166 is currently estimated to expire on March 26, 2039, while the Japan patent application, if granted, is currently estimated to expire on February 20, 2038. National stage applications are also pending in Australia, Brazil, Canada, China, European Union, Hong Kong, India, New Zealand, and Russia.
| -21- |
◌ A U.S. patent application (U.S. 15/932,387) and a PCT patent application (PCT/US2018/00085), titled “Albumin Binding Domain Fusion Proteins” originally received an application filing date of February 20, 2018, which is four days after the one-year anniversary of the filing date of U.S. provisional patent applications U.S. 62/459,975 and U.S. 62/459,981 to which both the U.S. patent application and the PCT patent application claim a priority benefit. A request to restore the priority benefit to the filing date of U.S. provisional patent applications U.S. 62/459,975 and U.S. 62/459,981 was granted for the U.S. patent application and PCT patent application. Subsequently, national phase patent applications were filed from the PCT patent application in Australia, Brazil, Canada, China, Europe, India, Japan, New Zealand and Russia. However, due to differences in the patent laws in these jurisdictions, the priority claims to U.S. 62/459,975 and U.S. 62/459,981 have thus far only been accepted in Australia, Europe, India, Japan, New Zealand, and Russia.
● US provisional application directed to anti-IL6-FHAB fusion proteins, including anti-IL6-FHAB, anti-IL6-FHAB-anti-TGFβ, and anti-IL6-FHAB-anti-IL8 fusion proteins; and methods of treatments using such fusion proteins was re-filed as US 63/245,702 on September 22, 2021. Due in large part to scientific challenges, the supportive data was not obtained within the one-year period after filing the provisional patent, and therefore, the patent was abandoned. However, recent technical breakthroughs will allow this abandoned patent to be re-filed in 2023.
● US provisional application directed to Antigen/Albumin Binding Domain Conjugates, and methods of treatments using such conjugates was re-filed as US 63/187,278 on May 11, 2021. Data in support of the provisional patent claims was not generated, and therefore, this patent was abandoned.
● US provisional application directed to Method of Treating Age-Related Frailty with Low-Dose IL6 filed June 4, 2021, as Application no. 63/197,097 and converted to a PCT patent in June 2022.
● US provisional application directed to Novel Antibody Drug Conjugate (ADC) Platform with an Albumin Binding Domain filed December 7, 2021, as Application no. 63/286,996.
● US provisional application directed to IL-12-Albumin-Binding Domain Fusion Protein Formulations and Methods of Use Thereof filed on May 27, 2022, as Application no. 63/346,368.
● US provisional application directed to Methods for the Treatment of Cancer with Recombinant IL-12 Albumin Binding Domain Fusion Proteins filed on Nov. 2, 2022, as Application no. 63/421,846.
With respect to our trademark portfolio, we received international registrational approval with the World Intellectual Property Office (WIPO) for the Sonnet BioTherapeutics and FHAB marks, each having an Effective Date of Sept. 17, 2020. Further, both marks were published by the European Union Intellectual Property Office (EUIPO), having Effective Dates of Nov. 30, 2020 and Dec. 6, 2020, respectively. In 2021, the USPTO issued Notices of Allowance for both marks, indicating that both applications have successfully completed the opposition period and have matured to registration with the submission acceptable Statements of Use. To that end, the USPTO issued a Notice of Allowance of the Statement of Use for each of the Sonnet BioTherapeutics and FHAB applications and we expect to receive the Certificates of Registration shortly.
● The Switzerland Trademark Office granted protection to the Sonnet BioTherapeutics and FHAB marks on Sept. 14, 2021, and Oct. 26, 2021, respectively, and are protected under International Trademark Registration nos. 1558330 and 1558471.
● The Canadian Intellectual Property Office granted protection to the Sonnet BioTherapeutics mark on June 8, 2022 and is protected under International Trademark Registration no. 1558330 while the FHAB mark is protected under International Trademark Registration no. 15584471, for which the 18-month opposition period began on Nov. 16, 2022.
| -22- |
● In addition to Switzerland and Canada, the Sonnet BioTherapeutics mark was also granted protection in Australia, European Union, Japan, Mexico, South Korea and the United Kingdom, in each case having a Registration no. of 1558330, an Effective Registration date of Sept. 17, 2020 and a renewal date of Sept. 17, 2030. Likewise, the FHAB mark was granted protection in Australia, China, European Union, Japan, Mexico, South Korea and the United Kingdom, in each case having a Registration no. of 1558471, a Granted Protection Date of Sept. 17, 2020 and renewal date of Sept. 17, 2030.
● Although the Sonnet BioTherapeutics mark was initially rejected in China due to potential non-use claims directed to certain competing companies, our law firm is quite confident that since the initial Class 42 rejection was successfully cancelled, two new trademark applications for this same mark were also registered and/or published in 2021 could also be overcome; however, we won’t be able to initiate non-use cancellation filings against these marks until 2025, which is the anticipated timeframe by which these pending class 42 applications are likely to become registered in China.
Employees
As of September 30, 2022, we had 12 full-time employees. None of our employees are represented by a labor union or covered by a collective bargaining agreement, and we believe our relationship with our employees is good. Additionally, we utilize independent contractors and other third parties to assist with various aspects of its business.
Government Regulation
The research, development, testing, manufacture, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of pharmaceutical products, including biological products, are extensively regulated by government authorities in the United States, at the federal, state and local level, and other countries and jurisdictions. Some jurisdictions also regulate the pricing of pharmaceutical products. The processes for obtaining marketing approvals in the United States and in foreign countries and jurisdictions, along with subsequent compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
Licensure and Regulation of Biologics in the United States
In the United States, biological products, or biologics, are regulated under the Public Health Service Act, or PHSA, and the Federal Food, Drug, and Cosmetic Act, or FDCA, and their implementing regulations. The failure to comply with the applicable requirements at any time during the product development process may subject an applicant to delays in the conduct of a study, regulatory review and approval, and/or administrative or judicial sanctions. These sanctions may include, without limitation, the FDA’s refusal to allow an applicant to proceed with clinical testing, refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, product recalls, product seizures, suspension of production or distribution, injunctions, fines, investigations and civil and criminal penalties. Biological product candidates must be granted a biological license by the FDA before they may be legally marketed in the United States.
| -23- |
The process required by the FDA to obtain a biological license in the United States generally involves the following:
● Completion of extensive nonclinical, or preclinical, laboratory tests and preclinical animal trials and applicable requirements for the humane use of laboratory animals and formulation studies in accordance with applicable regulations, including good laboratory practices, or GLPs;
● Submission to the FDA of an investigational new drug, or IND, application prior to initiation of any human clinical trials. Permission to proceed must be received before the beginning of such trials;
● Performance of adequate and well-controlled human clinical trials to establish the safety, potency and purity of the product candidate for each proposed indication, in accordance with the FDA’s regulation generally referred to as the good clinical practices, or GCP and any additional requirements for the protection of human research subjects and their health information, to establish the safety and efficacy of the proposed biological product for its intended use. The FDA may also impose clinical holds on biological product candidate at any time before or during our clinical trials due to safety concerns or non-compliance. If the FDA imposes a clinical hold, trials may not recommence without FDA authorization and then only under terms authorized by the FDA;
● Preparation and submission to the FDA of a Biologic License Application, or BLA, for a biologic product requesting marketing for one or more proposed indications, including submission of detailed information on the manufacture and composition of the product in clinical development and proposed labeling;
● Review of the product by an FDA advisory committee, as determined by the FDA review division;
● Satisfactory completion of one or more FDA inspections of the manufacturing facility or facilities, including those of third parties, at which the product, or components thereof, are produced to assess compliance with current Good Manufacturing Practices, or cGMP, requirements and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity;
● Satisfactory completion of one or more FDA audits of the clinical study sites to assure compliance with GCPs, and the integrity of clinical data in support of the BLA;
● Payment of user fees and securing FDA approval of the BLA and licensure of the new biologic product;
● Compliance with any post-approval requirements, including the potential requirement to implement a Risk Evaluation and Mitigation Strategy, or REMS, and any post-approval studies required by the FDA.
Nonclinical Studies and Investigational New Drug Application
Each product candidate must undergo nonclinical testing before testing in humans. These tests include laboratory evaluations of product chemistry, formulation and stability, as well as animal studies to evaluate the potential for activity and toxicity and must be conducted in compliance with applicable regulations. The results of the nonclinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND application. The IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions about the product or conduct of the proposed clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In that case, the IND sponsor and the FDA must resolve any outstanding FDA concerns before the clinical trial can begin.
Submission of the IND may result in the FDA not allowing the trial to commence or on the terms originally specified by the sponsor in the IND. If the FDA raises concerns or questions, it may choose to impose clinical holds on biological product candidates at any time before or during clinical trials due to safety concerns or non-compliance. If the FDA imposes a clinical hold, trials may not recommence without FDA authorization and only under terms authorized by the FDA.
| -24- |
Human Clinical Trials in Support of a BLA
Clinical trials involve the administration of the investigational product candidate to healthy volunteers or patients with the disease to be treated under the supervision of a qualified principal investigator in accordance with GCP requirements. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. A sponsor who wishes to conduct a clinical trial outside the United States may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. If a foreign clinical trial is not conducted under an IND, the sponsor may submit data from the clinical trial to the FDA in support of the BLA so long as the clinical trial is well-designed and well-conducted in accordance with GCP, including review and approval by an independent ethics committee, and the FDA is able to validate the study data through an onsite inspection, if necessary.
Further, each clinical trial must be reviewed and approved by an institutional review board, or IRB, either centrally or individually at each institution at which the clinical trial will be conducted or, for trials conducted outside of the United States, by an independent ethics committee referred to above. The IRB will consider, among other things, clinical trial design, patient informed consent, ethical factors and the safety of human subjects. An IRB must operate in compliance with FDA regulations. The FDA, IRB, or the clinical trial sponsor may suspend or discontinue a clinical trial at any time for various reasons, including a finding that the clinical trial is not being conducted in accordance with FDA requirements or the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive GCP rules and the requirements for informed consent. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group may recommend continuation of the study as planned, changes in study conduct, or cessation of the study at designated check points based on access to certain data from the study.
Clinical trials typically are conducted in three sequential phases that may overlap or be combined. Additional studies may be required after approval.
● Phase 1: the biological product candidate is initially introduced into healthy human volunteers and tested for safety. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients, such as cancer patients.
● Phase 2: the biological product candidate is evaluated in a limited patient population to identify possible adverse effects and safety risks, preliminary evaluate the efficacy of the product for specific targeted diseases and determine dosage tolerance, optimal dosage and dosing schedule.
● Phase 3: Clinical trials are undertaken to further evaluate dosage, clinical efficacy, potency and safety in an expanded patient population and geographically dispersed clinical study sites. These trials are intended to establish the overall risk/benefit ratio of the product and provide adequate basis for product labelling.
● Phase 4: post-approval clinical trials, or Phase 4 clinical trials, may be conducted after initial marketing approval. They provide additional experience for the treatment of patients in the intended therapeutic indication, particularly for long-term safety follow-up. If the FDA approves a product while a company has ongoing clinical trials that were not necessary for approval, a company may be able to use the data from these clinical trials to meet all or part of any Phase 4 clinical trial requirement or to request a change in the product labeling. Failure to exhibit due diligence with regard to conducting required Phase 4 clinical trials could result in withdrawal of approval for products.
Compliance with cGMP Requirements
Before approving a BLA, the FDA will typically inspect the facility(ies) where the product is manufactured to ensure full compliance of the manufacturing processes and facilities with cGMP requirements and consistent production with required specifications. Manufacturers and others involved in the manufacture and distribution of products must also register their establishments with the FDA and certain state agencies. Both domestic and foreign manufacturing establishments must register and provide additional information to the FDA upon their initial participation in the manufacturing process. Any product manufactured by or imported from a facility that has not registered is deemed misbranded under the FDCA. Establishments may be subject to periodic unannounced inspections by government authorities. Manufacturers may have to provide records regarding their establishments.
| -25- |
Review and Approval of a BLA
Results of product candidate development, nonclinical testing and clinical trials are submitted to the FDA as part of a BLA requesting a license to market the product. The BLA must contain extensive and detailed information on the manufacturing and composition of the product and proposed labeling as well as payment of a user fee. The FDA has 60 days after submission of the application to conduct an initial review to determine whether the BLA is sufficient to accept for filing. Once the submission has been accepted for filing, the FDA begins its in-depth review. The FDA has twelve months in which to complete its initial review of a standard application (or six months for a priority review) and respond to the applicant. The FDA does not always meet its goal dates and the review process may be significantly extended by FDA requests for additional information or clarification. The review process and the goal date may be extended by three months if the FDA requests or if the applicant otherwise provides additional information or clarification regarding information already provided in the submission within the last three months before the goal date.
On the basis of the FDA’s evaluation of the application and accompanying information, the FDA may issue an approval letter, denial letter, or a complete response letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. Under the PHSA, the FDA may approve a BLA if it determines that the product is safe, pure and potent and the facility where the product will be manufactured meets standards designed to ensure that it continues to be safe, pure and potent. If the application is not approved, the FDA may issue a complete response letter, which will contain the conditions that must be met in order to secure final approval of the application, and when possible, will outline recommended actions the sponsor might take to obtain approval of the application. Sponsors that receive a complete response letter may submit to the FDA information that represents a complete response to the issues identified by the FDA. Such resubmissions are classified under the Prescription Drug User Fee Act, or PDUFA, as either Class 1 or Class 2, based on the information submitted by an applicant in response to an action letter. Under the goals and policies agreed to by the FDA under PDUFA, the FDA has two months to review a Class 1 resubmission and six months to review a Class 2 resubmission. The FDA will not approve an application until issues identified in the complete response letter have been addressed. The FDA issues a denial letter if it determines that the establishment or product does not meet the agency’s requirements.
The FDA may also refer the application to an advisory committee for review, evaluation and non-binding recommendation as to whether the application should be approved. In particular, the FDA may refer applications for novel biologic products or biologic products that present difficult questions of safety or efficacy to an advisory committee.
If the FDA approves a new product, the FDA may limit its approved indications for use as well as require that contraindications, warnings or precautions be included in the product labeling. In addition, the FDA may call for post-approval studies, including Phase 4 clinical trials, to further assess the product’s safety after approval. The FDA may also require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, including REMS, to help ensure that the benefits of the product outweigh the potential risks. The FDA may prevent or limit further marketing of a product based on the results of post-market studies or surveillance programs. After approval, many types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Fast Track, Breakthrough Therapy and Priority Review Designations
The FDA is authorized to designate certain products for expedited review if they are intended to address an unmet medical need in the treatment of a serious or life-threatening disease or condition. These programs are referred to as (i) fast track designation, (ii) breakthrough therapy designation and (iii) priority review designation.
| -26- |
● Fast Track Review: The FDA may designate a product for fast track review if it is intended (alone or in combination with one or more other products) for the treatment of a serious or life-threatening disease or condition, and it demonstrates the potential to address unmet medical needs for such a disease or condition. Sponsors may have greater interactions with the FDA and the FDA may initiate review of sections of a fast track product’s application before the application is complete. The sponsor must also provide, and the FDA must approve, a schedule for the submission of the remaining information and the sponsor must pay applicable user fees. However, the FDA’s time period goal for reviewing a fast track application does not begin until the last section of the application is submitted. Fast track designation may be withdrawn by the FDA.
● Breakthrough Therapy: A product may be designated as a breakthrough therapy and be eligible for expedited review if it is intended, alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA may take certain actions with respect to breakthrough therapies.
● Priority Review: The FDA may designate a product for priority review if such product treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness when compared with other available therapies. This assessment is made by the FDA on a case-by-case basis. A priority designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA’s goal for taking action on a marketing application from 10 to six months.
Accelerated Approval Pathway
The FDA may grant accelerated approval to a product for a serious or life-threatening condition that provides meaningful therapeutic advantage to patients over existing treatments based upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit. The FDA may also grant accelerated approval for such a condition when the product has an effect on an intermediate clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality, or IMM, and that is reasonably likely to predict an effect on IMM or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. Products granted accelerated approval must meet the same statutory standards for safety and effectiveness as those granted traditional approval.
For the purposes of accelerated approval, a surrogate endpoint is a marker, such as a laboratory measurement, radiographic image, physical sign, or other measure that is thought to predict clinical benefit but is not itself a measure of clinical benefit. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. An intermediate clinical endpoint is a measurement of a therapeutic effect that is considered reasonably likely to predict the clinical benefit of a product, such as an effect on IMM. The accelerated approval pathway is most often used in settings in which the course of a disease is long, and an extended period of time is required to measure the intended clinical benefit of a product, even if the effect on the surrogate or intermediate clinical endpoint occurs rapidly. Thus, accelerated approval has been used extensively in the development and approval of products for treatment of a variety of cancers in which the goal of therapy is generally to improve survival or decrease morbidity and the duration of the typical disease course requires lengthy and sometimes large trials to demonstrate a clinical or survival benefit.
The accelerated approval pathway is usually contingent on a sponsor’s agreement to conduct, in a diligent manner, additional post-approval confirmatory studies to verify and describe the product’s clinical benefit. As a result, a product candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, would allow the FDA to withdraw the product from the market on an expedited basis. All promotional materials for product candidates approved under accelerated regulations are subject to prior review by the FDA.
| -27- |
Post-Approval Regulation
Even if regulatory approval is granted, a marketed product is subject to continuing comprehensive requirements under federal, state and foreign laws and regulations, including requirements and restrictions regarding adverse event reporting, recordkeeping, marketing, and compliance with cGMP. Adverse events reported after approval of a drug can result in additional restrictions on the use of a marketed product or requirements for additional post-marketing studies or clinical trials.
Maintaining substantial compliance with applicable federal, state and local statutes and regulations requires the expenditure of substantial time and financial resources. Rigorous and extensive FDA regulation of biological products continues after approval, particularly with respect to cGMP requirements. Biological product manufacturers and other entities involved in the manufacture and distribution of approved biological products are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP requirements and other laws. We will rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of any products that we may commercialize. Manufacturers of our products are required to comply with applicable requirements in the cGMP regulations, including quality control and quality assurance and maintenance of records and documentation. Other post-approval requirements applicable to biological products include record-keeping requirements, reporting of adverse effects and reporting updated safety and efficacy information.
Discovery of previously unknown problems or the failure to comply with the applicable regulatory requirements relating to the manufacturer or promotion of an approved product may result in restrictions on the marketing of a product or withdrawal of the product from the market as well as significant administrative, civil or criminal sanctions.
Orphan Drug Designation
Orphan drug designation in the United States is designed to encourage sponsors to develop products intended for rare diseases or conditions. In the United States, a rare disease or condition is statutorily defined as a condition that affects fewer than 200,000 individuals in the United States or that affects more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available the product for the disease or condition will be recovered from sales of the product in the United States.
Orphan drug designation qualifies a company for tax credits and market exclusivity for seven years following the date of the product’s marketing approval if granted by the FDA. An application for designation as an orphan product can be made any time prior to the filing of an application for approval to market the product. A product may be designated as an orphan drug by the FDA Office of Orphan Products Development, or OOPD, based on an acceptable application. The product must then go through the review and approval process like any other product. Orphan drug designations may be revoked based on a change in the incidence of the disease.
A sponsor may request orphan drug designation of a previously unapproved product or a new orphan indication for an already marketed product. In addition, a sponsor of a product that is otherwise the same product as an already approved orphan drug may seek and obtain orphan drug designation for the subsequent product for the same rare disease or condition if it can present a plausible hypothesis that its product may be clinically superior to the first drug. More than one sponsor may receive orphan drug designation for the same product for the same rare disease or condition, but each sponsor seeking orphan drug designation must file a complete request for designation.
The period of exclusivity begins on the date that the marketing application is approved by the FDA and applies only to the indication for which the product has been designated. The FDA may approve a second application for the same product for a different use or a second application for a clinically superior version of the product for the same use. The FDA cannot, however, approve the same product made by another manufacturer for the same indication during the market exclusivity period unless it has the consent of the sponsor or the sponsor is unable to provide sufficient quantities.
| -28- |
Pediatric Research
Under the Pediatric Research Equity Act, certain applications for approval must include an assessment, generally based on clinical study data, of the safety and effectiveness of the subject drug in relevant pediatric populations. The FDA may waive or defer the requirement for a pediatric assessment, either at the company’s request or by the FDA’s initiative. The FDA may determine that a Risk Evaluation and Mitigation Strategy are necessary to ensure that the benefits of a new product outweigh its risks. REMS may include various elements, ranging from a medication guide or patient package insert to limitations on who may prescribe or dispense the drug, depending on what the FDA considers necessary for the safe use of the drug. Sponsors are required to submit an initial pediatric study plan to their IND after their end-of-phase 2 meeting with the FDA
Regulation and Procedures Governing Approval of Medicinal Products in the European Union
In order to market any product outside of the United States, a company also must comply with numerous regulatory requirements of other countries and jurisdictions. Whether or not it obtains FDA approval for a product, an applicant will need to obtain the necessary approvals by the comparable foreign regulatory authorities before it can initiate clinical trials or marketing of the product in those countries or jurisdictions.
Clinical Trial Approval
Pursuant to the currently applicable Clinical Trials Directive 2001/20/EC and the Directive 2005/28/EC on GCP, a system for the approval of clinical trials in the European Union has been implemented through national legislation of the Member States. Under this system, an applicant must obtain approval from the competent national authority of a European Union Member State in which the clinical trial is to be conducted or in multiple Member States if the clinical trial is to be conducted in a number of Member States. Furthermore, the applicant may only start a clinical trial at a specific study site after the independent ethics committee has issued a favorable opinion. The clinical trial application, or CTA, must be accompanied by an investigational medicinal product dossier with supporting information prescribed by Directive 2001/20/EC and Directive 2005/28/EC and corresponding national laws of the Member States and further detailed in applicable guidance documents.
In April 2014, the European Union adopted a new Clinical Trials Regulation (EU) No 536/2014, which is set to replace the current Clinical Trials Directive 2001/20/EC. It is expected that the new Clinical Trials Regulation will apply in 2019 or 2020. It will overhaul the current system of approvals for clinical trials in the European Union. Specifically, the new regulation, which will be directly applicable in all Member States, aims at simplifying and streamlining the approval of clinical trials in the European Union. For instance, the new Clinical Trials Regulation provides for a streamlined application procedure using a single entry point and strictly defined deadlines for the assessment of clinical trial applications.
Marketing Authorization
To obtain a marketing authorization for a product under the European Union regulatory system, an applicant must submit an MAA, either under a centralized procedure administered by the EMA or one of the procedures administered by competent authorities in European Union Member States (decentralized procedure, national procedure, or mutual recognition procedure). A marketing authorization may be granted only to an applicant established in the European Union. An applicant must demonstrate compliance with all measures included in an EMA-approved Pediatric Investigation Plan, or PIP, covering all subsets of the pediatric population, unless the EMA has granted a product-specific waiver, class waiver, or a deferral for one or more of the measures included in the PIP.
The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid for all European Union Member States. It is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy products and products with a new active substance indicated for the treatment of certain diseases, including products for the treatment of cancer. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients, the centralized procedure may be optional.
| -29- |
Under the centralized procedure, the Committee for Medicinal Products for Human Use, or the CHMP, established at the EMA is responsible for conducting the assessment of a product to define its risk/benefit profile. Under the centralized procedure, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated evaluation may be granted by the CHMP in exceptional cases, when a medicinal product is of major interest from the point of view of public health and, in particular, from the viewpoint of therapeutic innovation.
Periods of Authorization and Renewals
A marketing authorization is valid for five years, in principle, and it may be renewed after five years on the basis of a reevaluation of the risk benefit balance by the EMA or by the competent authority of the authorizing Member State. Once renewed, the marketing authorization is valid for an unlimited period, unless the European Commission or the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal period. Any authorization that is not followed by the placement of the drug on the European Union market (in the case of the centralized procedure) or on the market of the authorizing Member State within three years after authorization ceases to be valid.
Regulatory Requirements after Marketing Authorization
Following approval, the holder of the marketing authorization is required to comply with a range of requirements applicable to the manufacturing, marketing, promotion and sale of the medicinal product. These include compliance with the European Union’s stringent pharmacovigilance or safety reporting rules, pursuant to which post-authorization studies and additional monitoring obligations can be imposed. In addition, the manufacturing of authorized products, for which a separate manufacturer’s license is mandatory, must also be conducted in strict compliance with the EMA’s GMP requirements and comparable requirements of other regulatory bodies in the European Union, which mandate the methods, facilities and controls used in manufacturing, processing and packing of drugs to assure their safety and identity. Finally, the marketing and promotion of authorized products, including industry-sponsored continuing medical education and advertising directed toward the prescribers of drugs and/or the general public, are strictly regulated in the European Union under Directive 2001/83EC, as amended.
Orphan Drug Designation and Exclusivity
Regulation (EC) No. 141/2000 and Regulation (EC) No. 847/2000 provide that a product can be designated as an orphan drug by the European Commission if its sponsor can establish: that the product is intended for the diagnosis, prevention or treatment of (1) a life-threatening or chronically debilitating condition affecting not more than five in ten thousand persons in the European Union when the application is made, or (2) a life-threatening, seriously debilitating or serious and chronic condition in the European Union and that without incentives it is unlikely that the marketing of the drug in the European Union would generate sufficient return to justify the necessary investment. For either of these conditions, the applicant must demonstrate that there exists no satisfactory method of diagnosis, prevention, or treatment of the condition in question that has been authorized in the European Union or, if such method exists, the drug has to be of significant benefit compared to products available for the condition. An orphan drug designation provides benefits such as fee reductions, regulatory assistance and the possibility to apply for a centralized European Union marketing authorization. Marketing authorization for an orphan drug leads to a ten-year period of market exclusivity. The market exclusivity period may however be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan drug designation.
Combination Products in the United States
Certain products, the combination products, may be comprised of components that would normally be regulated under different types of regulatory authorities and frequently by different centers at the FDA. A combination product may be (i) a product comprised of two or more regulated components that are physically, chemically, or otherwise combined or mixed and produced as a single entity; (ii) two or more separate products packaged together in a single package or as a unit and comprised of drug and device products, device and biological products, or biological and drug products; (iii) drug, or device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, or device, or biological product where both are required to achieve the intended use, indication, or effect and where upon approval of the proposed product the labeling of the approved product would need to be changed, e.g., to reflect a change in intended use, dosage form, strength, route of administration, or significant change in dose; or (iv) any investigational drug, device, or biological product packaged separately that according to its proposed labeling is for use only with another individually specified investigational drug, device, or biological product where both are required to achieve the intended use, indication, or effect. The FDA is charged with assigning a center with primary jurisdiction, or a lead center, for review of a combination product, this determination being based on the “primary mode of action” of the combination product. Sponsors may request a jurisdiction determination by submitting a Request for Designation to the office of Combination Drug Products.
| -30- |
Merger with Chanticleer and Acquisition of Relief
This Annual Report on Form 10-K is filed by Sonnet BioTherapeutics Holdings, Inc. (“Sonnet Holdings,” “we,” “us,” “our,” or the “Company”), formerly known as Chanticleer Holdings, Inc. Until March 31, 2020, the Company was in the business of owning, operating and franchising fast casual dining concepts domestically and internationally. As previously disclosed, on April 1, 2020, the Company completed its merger transaction with Sonnet BioTherapeutics, Inc. (“Sonnet”), pursuant to which Sonnet became a wholly-owned subsidiary of the Company (the “Merger”). On April 1, 2020, in connection with the Merger, the Company changed its name to “Sonnet BioTherapeutics Holdings, Inc.” Sonnet was incorporated as a New Jersey corporation on April 6, 2015.
The Merger was treated by the Company as a reverse merger and accounted for as a reverse recapitalization in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). For accounting purposes, Sonnet is considered to have acquired the Company.
In connection with and prior to the Merger, the Company contributed and transferred to Amergent Hospitality Group, Inc. (“Amergent”), a newly formed, wholly owned subsidiary of the Company, all of the assets and liabilities relating to the Company’s restaurant business. The dividend, which together with the contribution and transfer of the Company’s restaurant business described above, is referred to as the “Spin-Off.” Prior to the Spin-Off, Amergent engaged in no business or operations.
As a result of the Spin-Off and the Merger, since April 1, 2020, the Company has operated through Sonnet and its direct and indirect subsidiaries and the ongoing business of the Company is the Sonnet business.
In addition, in connection with and prior to the Merger, on April 1, 2020, Sonnet completed its acquisition of the global development rights for Atexakin Alfa (low dose formulation of Interleukin-6, IL-6, now “SON-080”) from Relief Therapeutics Holding SA (“Relief Holding”) through its acquisition of Relief Holding’s wholly-owned subsidiary, Relief Therapeutics SA (“Relief”), in exchange for the issuance to Relief Holding of shares of Sonnet common stock that converted into an aggregate of 757,933 shares of Company common stock in the Merger.
Corporate and Available Information
The Company was organized on October 21, 1999, under its original name, Tulvine Systems, Inc., under the laws of the State of Delaware. On April 25, 2005, Tulvine Systems, Inc. formed a wholly owned subsidiary, Chanticleer Holdings, Inc., and on May 2, 2005, Tulvine Systems, Inc. merged with, and changed its name to, Chanticleer Holdings, Inc. On April 1, 2020, the Company completed its business combination with Sonnet BioTherapeutics, Inc. (“Sonnet”), in accordance with the terms of the Agreement and Plan of Merger, dated as of October 10, 2019, as amended, by and among the Company, Sonnet and Biosub Inc., a wholly-owned subsidiary of the Company (“Merger Sub”) (the “Merger Agreement”), pursuant to which Merger Sub merged with and into Sonnet, with Sonnet surviving as a wholly owned subsidiary of the Company (the “Merger”). Under the terms of the Merger Agreement, the Company issued shares of common stock to Sonnet’s stockholders at an exchange rate of 0.106572 shares for each share of Sonnet common stock outstanding immediately prior to the Merger. In connection with the Merger, the Company changed its name from “Chanticleer Holdings, Inc.” to “Sonnet BioTherapeutics Holdings, Inc.,” and the business conducted by the Company became the business conducted by Sonnet.
Our principal executive offices are located at 100 Overlook Center, Suite 102, Princeton, New Jersey 08540. Our telephone number is (609) 375-2227 and the corporate website address is https://www.sonnetbio.com/. We included the website address in this annual report on Form 10-K only as an inactive textual reference and do not intend it to be an active link to our website. The information on the website is not incorporated by reference in this annual report on Form 10-K.
This annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and all amendments to those reports, as well as other documents we file with the U.S. Securities and Exchange Commission (“SEC”), are available free of charge through the Investors section of our website as soon as reasonably practicable after such material is electronically filed with or furnished to the SEC. The public can obtain documents that we file with the SEC at www.sec.gov.
| -31- |
Item 1A. Risk Factors.
An investment in our common stock involves a high degree of risk including the risk of a loss of your entire investment. You should carefully consider the risks and uncertainties described below and the other information contained in this report and the other reports filed by us with the SEC. The risks set forth below are not the only ones facing us. Additional risks and uncertainties may exist that could also adversely affect our business, operations and financial condition. If any of the following risks actually materialize, our business, financial condition and/or operations could suffer. In such event, the value of our common stock could decline, and you could lose all or a substantial portion of the money that you pay for our common stock.
Summary of Risk Factors
| ● | We have a history of significant operating losses and expect to incur significant and increasing losses for the foreseeable future, and we may never achieve or maintain profitability. | |
| ● | Our recurring losses from operations have raised substantial doubt regarding our ability to continue as a going concern. | |
| ● | We will need substantial additional funding, and if we are unable to raise capital when needed, we could be forced to delay, reduce or eliminate our product discovery and development programs or commercialization efforts. | |
| ● | The coronavirus COVID-19 pandemic or the widespread outbreak of any other communicable disease could materially and adversely affect our business, financial condition and results of operations. | |
| ● | We are substantially dependent on the success of our internal development programs and our product pipeline candidates may not successfully complete clinical trials, receive regulatory approval or be successfully commercialized. | |
| ● | We are at a very early stage in our development efforts, our product candidates represent a new category of medicines and may be subject to heightened regulatory scrutiny until they are established as a therapeutic modality. | |
| ● | Even if we complete the necessary preclinical studies and clinical trials, the marketing approval process is expensive, time consuming and uncertain and may prevent us or any collaborators from obtaining approvals for the commercialization of some or all of our product candidates. As a result, we cannot predict when or if, and in which territories, we, or any collaborators, will obtain marketing approval to commercialize a product candidate. | |
| ● | We face significant competition and if our competitors develop and market products that are more effective, safer or less expensive than the product candidates we develop, our commercial opportunities will be negatively impacted. | |
| ● | The commercial success of any current or future product candidate will depend upon the degree of market acceptance by physicians, patients, payors and others in the medical community. | |
| ● | For certain product candidates, we may depend on development and commercialization collaborators to develop and conduct clinical trials with, obtain regulatory approvals for, and if approved, market and sell product candidates. If such collaborators fail to perform as expected, the potential for us to generate future revenue from such product candidates would be significantly reduced and our business would be harmed. | |
| ● | We will rely on third parties, including independent clinical investigators and CROs, to conduct and sponsor some of the clinical trials of our product candidates. Any failure by a third party to meet its obligations with respect to the clinical development of our product candidates may delay or impair our ability to obtain regulatory approval for our product candidates. | |
| ● | If we are unable to obtain and maintain patent and other intellectual property protection for our products and product candidates, or if the scope of the patent and other intellectual property protection obtained is not sufficiently broad, our competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize our products and product candidates may be adversely affected. | |
| ● | We expect to expand our organization, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations. | |
| ● | We do not expect to pay cash dividends in the foreseeable future and therefore investors should not anticipate cash dividends on their investment. |
| -32- |
Risks Related to Our Financial Position and Need for Additional Capital
We have a history of significant operating losses and expect to incur significant and increasing losses for the foreseeable future, and we may never achieve or maintain profitability.
We do not expect to generate revenue or profitability that is necessary to finance our operations in the short term. Our net losses for the years ended September 30, 2022 and 2021 were $29.7 million and $25.0 million, respectively. As of September 30, 2022, we had an accumulated deficit of $91.4 million.
To date, we have not commercialized any products or generated any revenues from the sale of products, and absent the realization of sufficient revenues from product sales, we may never attain profitability in the future. We have devoted substantially all of our financial resources and efforts to research and development, including preclinical studies and our clinical trials. Our net losses may fluctuate significantly from quarter to quarter and year to year. Net losses and negative cash flows have had, and will continue to have, an adverse effect on our shareholders’ (deficit) equity and working capital.
We anticipate that our expenses will increase substantially if and as we:
● continue to develop and conduct clinical trials with respect to our lead product candidate, SON-080, and our other product candidates;
● initiate and continue research, preclinical and clinical development efforts for any future product candidates;
● seek to discover and develop additional product candidates and further expand our clinical product pipeline;
● seek marketing and regulatory approvals for any product candidates that successfully complete clinical trials;
● require the manufacture of larger quantities of product candidates for clinical development and, potentially, commercialization;
● maintain, expand and protect our intellectual property portfolio;
● expand our research and development infrastructure, including hiring and retaining additional personnel, such as clinical, quality control and scientific personnel;
● establish sales, marketing, distribution and other commercial infrastructure in the future to commercialize products for which we obtain marketing approval, if any;
● add operational, financial and management information systems and personnel, including personnel to support our product development and commercialization and help us comply with our obligations as a public company; and
● add equipment and physical infrastructure to support our research and development.
Our ability to become and remain profitable depends on our ability to license our products and generate revenue. Generating product revenue will depend on our ability to obtain marketing approval for, and successfully commercialize, one or more of our product candidates.
| -33- |
Successful commercialization will require achievement of key milestones, including completing clinical trials of our product candidates, obtaining marketing approval for these product candidates, manufacturing, marketing and selling those products for which we, or any collaborators, may obtain marketing approval, satisfying any post-marketing requirements and obtaining reimbursement for our products from private insurance or government payors. Because of the uncertainties and risks associated with these activities, we are unable to accurately predict the timing and amount of revenues, and if or when we might achieve profitability. We and any collaborators may never succeed in these activities and, even if we do, or any collaborators do, we may never generate revenues that are large enough for us to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis.
Our failure to become and remain profitable would depress the market price of our common stock and could impair our ability to raise capital, expand our business, diversify our product offerings or continue our operations. If we continue to suffer losses, investors may not receive any return on their investment and may lose their entire investment.
Our limited operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
Our business commenced operations in 2015. Our operations to date have been limited to financing and staffing our company, developing our technology, conducting preclinical research and early-stage clinical trials for our product candidates and pursuing strategic collaborations to advance our product candidates. We have not yet demonstrated an ability to successfully conduct late-stage clinical trials, obtain marketing approvals, manufacture a commercial-scale product, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Accordingly, you should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by companies in the early stages of development, especially clinical-stage biopharmaceutical companies such as ours. Any predictions you make about our future success or viability may not be as accurate as they would be if we had a longer operating history or a history of successfully developing and commercializing pharmaceutical products.
We may encounter unforeseen expenses, difficulties, complications, delays and other known or unknown factors in achieving our business objectives. We will eventually need to transition from a company with a development focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
We expect our financial condition and operating results to continue to fluctuate significantly from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. Accordingly, you should not rely upon the results of any quarterly or annual periods as indications of future operating performance.
Our recurring losses from operations have raised substantial doubt regarding our ability to continue as a going concern.
We have incurred recurring losses and negative cash flows from operations activities since inception and we expect to generate losses and negative cash flows from operations for the foreseeable future primarily due to research and development costs for our potential product candidates. As of September 30, 2022, we had cash of $3.1 million and stockholders’ deficit of $2.5 million. We believe our cash at September 30, 2022, together with the $4.6 million in net proceeds raised through our at-the-market offering with BTIG, LLC subsequent to September 30, 2022 and through the date of this report will fund our projected operations into March 2023. Substantial additional financing will be needed by us to fund our operations. These factors raise substantial doubt about our ability to continue as a going concern. The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
We will require additional capital in the future through equity or debt financings, partnerships, collaborations, or other sources to carry out our planned development activities. If additional capital is not secured when required, we may need to delay or curtail our operations until such funding is received. Various internal and external factors will affect whether and when our product candidates become approved for marketing and successful commercialization. The regulatory approval and market acceptance of our products candidates, length of time and cost of developing and commercializing these product candidates and/or failure of them at any stage of the approval process will materially affect our financial condition and future operations.
| -34- |
Operations since inception have consisted primarily of organizing us, securing financing, developing its technologies through performing research and development and conducting preclinical studies. We face risks associated with companies whose products are in development. These risks include the need for additional financing to complete its research and development, achieving its research and development objectives, defending its intellectual property rights, recruiting and retaining skilled personnel, and dependence on key members of management.
Our ability to continue as a going concern is dependent on our ability to raise additional equity or debt capital or spin-off non-core assets to raise additional cash. Should we be unable to raise sufficient additional capital, we may be required to undertake cost-cutting measures including delaying or discontinuing certain clinical activities.
The source, timing and availability of any future financing will depend principally upon market conditions, and, more specifically, on the progress of our clinical development programs. Funding may not be available when needed, at all, or on terms acceptable to us. Lack of necessary funds may require us, among other things, to delay, scale back or eliminate some or all of our planned clinical trials. These factors among others create a substantial doubt about our ability to continue as a going concern.
While the potential economic impact brought by, and the duration of, COVID-19, discussed further below, may be difficult to assess or predict, a widespread pandemic could result in significant disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect our liquidity. In addition, a recession or market correction resulting from the spread of COVID-19 could materially affect our business and the value of our common shares.
We will need substantial additional funding, and if we are unable to raise capital when needed, we could be forced to delay, reduce or eliminate our product discovery and development programs or commercialization efforts.
Developing pharmaceutical products, including conducting preclinical studies and clinical trials, is a very time-consuming, expensive and uncertain process that takes years to complete. For example, for the years ended September 30, 2022 and 2021, we used $27.7 million and $22.6 million, respectively, in net cash for our operating activities, substantially all of which related to research and development activities. We expect our expenses to increase in connection with our ongoing activities, particularly as we initiate new clinical trials of, initiate new research and preclinical development efforts for and seek marketing approval for, our current product candidates or any future product candidates. In addition, if we obtain marketing approval for any of our product candidates, we may incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution to the extent that such sales, marketing, manufacturing and distribution are not the responsibility of a collaborator. Furthermore, as a result of the Merger, we will continue to incur significant costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we may be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts.
We will be required to expend significant funds in order to advance the development of the product candidates in our pipeline, as well as other product candidates we may seek to develop. In addition, while we may seek one or more collaborators for future development of our product candidates, we may not be able to enter into a collaboration for any of our product candidates for such indications on suitable terms, on a timely basis or at all. In any event, our existing cash will not be sufficient to fund all of the efforts that we plan to undertake or to fund the completion of development of any of our product candidates. Accordingly, we will be required to obtain further funding through public or private equity offerings, debt financings, collaborations and licensing arrangements or other sources. We do not have any committed external source of funds. Adequate additional financing may not be available to us on acceptable terms, or at all. Our failure to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategy.
| -35- |
Our estimate may prove to be wrong, and we could use our available capital resources sooner than we currently expect. Further, changing circumstances, some of which may be beyond our control, could cause us to consume capital significantly faster than we currently anticipate, and we may need to seek additional funds sooner than planned. Our future funding requirements, both short-term and long-term, will depend on many factors, including:
● the scope, progress, timing, costs and results of clinical trials of, and research and preclinical development efforts for, our current and future product candidates;
● our ability to enter into, and the terms and timing of, any collaborations, licensing or other arrangements;
● our ability to identify one or more future product candidates for our pipeline;
● the number of future product candidates that we pursue and their development requirements;
● the outcome, timing and costs of seeking regulatory approvals;
● the costs of commercialization activities for any of our product candidates that receive marketing approval to the extent such costs are not the responsibility of any collaborators, including the costs and timing of establishing product sales, marketing, distribution and manufacturing capabilities;
● the receipt of marketing approval, revenue, if any, received from commercial sales of our current and future product candidates;
● our headcount growth and associated costs as we expand our research and development and establish a commercial infrastructure;
● the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights including enforcing and defending intellectual property related claims; and
● the costs of operating as a public company.
Raising additional capital may cause dilution to our existing shareholders, restrict our operations or cause us to relinquish valuable rights.
We may seek additional capital through a combination of public and private equity offerings, debt financings, strategic partnerships and alliances, licensing arrangements or monetization transactions. To the extent that we raise additional capital through the sale of equity, convertible debt securities or other equity-based derivative securities, your ownership interest will be diluted and the terms may include liquidation or other preferences that adversely affect your rights as a shareholder. Any indebtedness we incur would result in increased fixed payment obligations and could involve restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Furthermore, the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our common stock to decline and existing shareholders may not agree with our financing plans or the terms of such financings. If we raise additional funds through strategic partnerships and alliances, licensing arrangements or monetization transactions with third parties, we may have to relinquish valuable rights to our technologies, or our product candidates, or grant licenses on terms unfavorable to us. Adequate additional financing may not be available to us on acceptable terms, or at all. If we are unable to raise additional funds when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
| -36- |
Risks Related to the Discovery, Development and Regulatory Approval of Our Product Candidates
The coronavirus COVID-19 pandemic or the widespread outbreak of any other communicable disease could materially and adversely affect our business, financial condition and results of operations.
We face risks related to health epidemics or outbreaks of communicable diseases, for example, the recent outbreak around the world of the highly transmissible and pathogenic coronavirus COVID-19. The outbreak of such communicable diseases could result in a widespread health crisis that could adversely affect general commercial activity and the economies and financial markets of many countries.
In December 2019, a novel strain of coronavirus, COVID-19, was reported to have surfaced in Wuhan, China and on March 11, 2020 was declared a pandemic by the World Health Organization. The extent to which COVID-19 may impact our preclinical and clinical trial operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the duration and geographic reach of the outbreak, the severity of COVID-19, and the effectiveness of actions to contain and treat COVID-19.
To date, many countries around the world imposed quarantines and restrictions on travel and mass gatherings to slow the spread of COVID-19 and have closed non-essential businesses. As countries and state and local jurisdictions continue to put restrictions in place, our ability to continue to operate our business may also be limited. Such events may result in a period of business, supply and drug product manufacturing disruption, and in reduced operations, any of which could materially affect our business, financial condition and results of operations.
This pandemic or outbreak could result in difficulty securing clinical trial site locations, CROs, and/or trial monitors and other critical vendors and consultants supporting the trial. In addition, outbreaks or the perception of an outbreak near a clinical trial site location could impact our ability to enroll patients. These situations, or others associated with Covid-19, could cause delays in our clinical trial plans and could increase expected costs, all of which could have a material adverse effect on our business and its financial condition.
In particular, manufacturing of our pipeline products (other than SON-1010) has been delayed by COVID-19 related supply chain issues, specifically supply of raw materials, including media, resins, and analytical kits, compounded by international shipping delays.
While the potential economic impact brought by, and the duration of, COVID-19 may be difficult to assess or predict, a widespread pandemic could result in significant disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect our liquidity. In addition, a recession or market correction resulting from the spread of COVID-19 could materially affect our business and the value of our common shares.
The COVID-19 outbreak may also affect the ability of our staff and the parties we work with to carry out our non-clinical, clinical, and drug manufacturing activities. We rely or may in the future rely on clinical sites, investigators and other study staff, consultants, independent contractors, contract research organizations and other third-party service providers to assist us in managing, monitoring and otherwise carrying out our nonclinical studies and clinical trials. We also rely or may in the future rely on consultants, independent contractors, contract manufacturing organizations, and other third-party service providers to assist us in managing, monitoring and otherwise carrying out our API production, formulation, and drug manufacturing activities. COVID-19 may affect the ability of any of these external people, organizations, or companies to devote sufficient time and resources to our programs or to travel to perform work for us.
Potential negative impacts of the COVID-19 outbreak on the conduct of current or future clinical studies include delays in gaining feedback from regulatory agencies, starting new clinical studies, and recruiting subjects to studies that are enrolling. The potential negative impacts also include inability to have study visits at study sites, incomplete collection of safety and efficacy data, and higher rates of drop-out of subjects from ongoing studies, delays in site entry of study data into the data base, delays in monitoring of study data because of restricted physical access to study sites, delays in site responses to queries, delays in data-base lock, delays in data analyses, delays in time to top-line data, and delays in completing study reports. New or worsening COVID-19 disruptions or restrictions could have the potential to further negatively impact our non-clinical studies, clinical trials, and drug manufacturing activities.
| -37- |
We are substantially dependent on the success of our internal development programs and our product pipeline candidates may not successfully complete clinical trials, receive regulatory approval or be successfully commercialized.
Our future success will depend heavily on the success of our internal development programs and of product candidates from our pipeline program.
Our ability to successfully commercialize our pipeline and our other product candidates will depend on, among other things, our ability to:
● successfully complete preclinical studies and clinical trials;
● receive regulatory approvals from the FDA, the EMA and other similar regulatory authorities;
● establish and maintain collaborations with third parties for the development and/or commercialization of our product candidates, or otherwise build and maintain strong development, sales, distribution and marketing capabilities that are sufficient to develop products and launch commercial sales of any approved products;
● obtain coverage and adequate reimbursement from payors such as government health care systems and insurance companies and achieve commercially attractive levels of pricing;
● secure acceptance of our product candidates from physicians, health care payors, patients and the medical community;
● produce, through a validated process, in manufacturing facilities inspected and approved by regulatory authorities, including the FDA, sufficiently large quantities of our product candidates to permit successful commercialization;
● manage our spending as expenses increase due to clinical trials and commercialization; and
● obtain and enforce sufficient intellectual property rights for any approved products and product candidates.
Of the large number of drugs in development in the pharmaceutical industry, only a small percentage result in the submission of a new drug application, or NDA, or biologics licensing application, or BLA, to the FDA and even fewer are approved for commercialization. Furthermore, even if we do receive regulatory approval to market our product candidates, any such approval may be subject to limitations on the indicated uses or patient populations for which we may market the product. Accordingly, even if we are able to obtain the requisite financing to continue to fund our development programs, we cannot assure you that our product candidates will be successfully developed or commercialized. If we are unable to develop, or obtain regulatory approval for, or, if approved, to successfully commercialize our product candidates, we may not be able to generate sufficient revenue to continue our business.
We are at a very early stage in our development efforts, our product candidates represent a new category of medicines and may be subject to heightened regulatory scrutiny until they are established as a therapeutic modality.
Our pipeline product candidates represent a new therapeutic modality of including engaging a Fully Human Albumin Binding Domain to deliver therapeutic products. Our product candidates may not demonstrate in patients any or all of the pharmacological benefits we believe they may possess. We have not yet succeeded and may never succeed in demonstrating efficacy and safety for these or any other product candidates in clinical trials or in obtaining marketing approval thereafter.
Regulatory authorities do not have experience with our product candidate and may require evidence of safety and efficacy that goes beyond what we have included in our development plans. In such a case, development of our product candidates may be more costly or time-consuming than expected, and our candidate products may not prove to be viable.
| -38- |
If we are unsuccessful in our development efforts, we may not be able to advance the development of our product candidates, commercialize products, raise capital, expand our business or continue our operations.
Our product candidates and those of any collaborators will need to undergo preclinical and clinical trials that are time-consuming and expensive, the outcomes of which are unpredictable, and for which there is a high risk of failure. If preclinical or clinical trials of our or their product candidates fail to satisfactorily demonstrate safety and efficacy to the FDA, the EMA and any other comparable regulatory authority, additional costs may be incurred or delays experienced in completing, the development of these product candidates, or their development may be abandoned.
The FDA in the United States, the EMA in the European Union and the European Economic Area, and other comparable regulatory authorities in other jurisdictions must approve new product candidates before they can be marketed, promoted or sold in those territories. We have not previously submitted an IND or BLA to the FDA or similar drug approval filings to comparable foreign regulatory authorities for any of our product candidates. We must provide these regulatory authorities with data from preclinical studies and clinical trials that demonstrate that our product candidates are safe and effective for a specific indication before they can be approved for commercial distribution. We cannot be certain that our clinical trials for our product candidates will be successful or that any of our product candidates will receive approval from the FDA, the EMA or any other comparable regulatory authority.
Preclinical studies and clinical trials are long, expensive and unpredictable processes that can be subject to extensive delays. We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. It may take several years and require significant expenditures to complete the preclinical studies and clinical trials necessary to commercialize a product candidate, and delays or failure are inherently unpredictable and can occur at any stage. We may also be required to conduct additional clinical trials or other testing of our product candidates beyond the trials and testing that we contemplate, which may lead to us incurring additional unplanned costs or result in delays in clinical development. In addition, we may be required to redesign or otherwise modify our plans with respect to an ongoing or planned clinical trial, and changing the design of a clinical trial can be expensive and time consuming. An unfavorable outcome in one or more trials would be a major setback for our product candidates and for us. An unfavorable outcome in one or more trials may require us to delay, reduce the scope of or eliminate one or more product development programs, which could have a material adverse effect on our business, financial position, results of operations and future growth prospects.
Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of marketing approval for our product candidates. The FDA, EMA or any other comparable regulatory authority may disagree with our clinical trial design and our interpretation of data from clinical trials, or may change the requirements for approval even after it has reviewed and commented on the design for our clinical trials.
In connection with clinical trials of our product candidates, we face a number of risks, including risks that:
● a product candidate is ineffective or inferior to existing approved products for the same indications;
● a product candidate causes or is associated with unacceptable toxicity or has unacceptable side effects;
● patients may die or suffer adverse effects for reasons that may or may not be related to the product candidate being tested;
● the results may not confirm the positive results of earlier trials;
● the results may not meet the level of statistical significance required by the FDA, the EMA or other relevant regulatory agencies to establish the safety and efficacy of our product candidates for continued trial or marketing approval; and
● our collaborators may be unable or unwilling to perform under their contracts.
| -39- |
Furthermore, we sometimes estimate for planning purposes the timing of the accomplishment of various scientific, clinical, regulatory and other product development objectives. These milestones may include our expectations regarding the commencement or completion of scientific studies, clinical trials, the submission of regulatory filings or commercialization objectives. From time to time, we may publicly announce the expected timing of some of these milestones, such as the completion of an ongoing clinical trial, the initiation of other clinical programs, the receipt of marketing approval or a commercial launch of a product. The achievement of many of these milestones may be outside of our control. All of these milestones are based on a variety of assumptions, which may cause the timing of achievement of the milestones to vary considerably from our estimates. If we fail to achieve milestones in the timeframes we expect, the commercialization of our product candidates may be delayed, we may not be entitled to receive certain contractual payments, which could have a material adverse effect on our business, financial position, results of operations and future growth prospects.
We may find it difficult to enroll patients in our clinical trials, which could delay or prevent us from proceeding with clinical trials of our product candidates.
Identifying and qualifying patients to participate in clinical trials of our product candidates is critical to our success. The timing of our clinical trials depends on our ability to recruit patients to participate as well as the completion of required follow-up periods. Patients may be unwilling to participate in our clinical trials because of negative publicity from adverse events related to novel therapeutic approaches, competitive clinical trials for similar patient populations, the existence of current treatments or for other reasons. Enrollment risks are heightened with respect to certain indications that we may target for one or more of our product candidates that may be rare diseases, which may limit the pool of patients that may be enrolled in our planned clinical trials. The timeline for recruiting patients, conducting trials and obtaining regulatory approval of our product candidates may be delayed, which could result in increased costs, delays in advancing our product candidates, delays in testing the effectiveness of our product candidates or termination of the clinical trials altogether.
We may not be able to identify, recruit and enroll a sufficient number of patients, or those with the required or desired characteristics, to complete our clinical trials in a timely manner. For example, due to the nature of the indications that we are initially targeting, patients with advanced disease progression may not be suitable candidates for treatment with our product candidates and may be ineligible for enrollment in our clinical trials. Therefore, early diagnosis in patients with our target diseases is critical to our success. Patient enrollment and trial completion is affected by factors including the:
● size of the patient population and process for identifying subjects;
● design of the trial protocol;
● eligibility and exclusion criteria;
● safety profile, to date, of the product candidate under study;
● perceived risks and benefits of the product candidate under study;
● perceived risks and benefits of our approach to treatment of diseases;
● availability of competing therapies and clinical trials;
● severity of the disease under investigation;
● degree of progression of the subject’s disease at the time of enrollment;
● proximity and availability of clinical trial sites for prospective subjects;
● ability to obtain and maintain subject consent;
● risk that enrolled subjects will drop out before completion of the trial;
| -40- |
● patient referral practices of physicians; and
● ability to monitor subjects adequately during and after treatment.
In addition, clinical development for pilot scale feasibility study of SON-080 is currently planned to take place outside of the U.S. Our ability to successfully initiate, enroll and complete a clinical trial in any foreign country is subject to numerous risks unique to conducting business in foreign countries, including:
● difficulty in establishing or managing relationships with academic partners or contract research organizations, or CROs, and physicians;
● different standards for the conduct of clinical trials;
● the absence in some countries of established groups with sufficient regulatory expertise for review of protocols related to our novel approach;
● our inability to locate qualified local consultants, physicians and partners; and
● the potential burden of complying with a variety of foreign laws, medical standards and regulatory requirements, including the regulation of pharmaceutical and biotechnology products and treatment.
If we have difficulty enrolling a sufficient number of patients to conduct our clinical trials as planned, we may need to delay, limit or terminate ongoing or planned clinical trials, any of which would have an adverse effect on our business, financial condition, results of operations and prospects.
Results of preclinical studies and early clinical trials may not be predictive of results of future clinical trials.
The outcome of preclinical studies and early clinical trials may not be predictive of the success of later clinical trials, and interim results of clinical trials do not necessarily predict success in the results of completed clinical trials. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials after achieving positive results in earlier development, and we could face similar setbacks. For example, the Phase IIa trial of SON-080 will be conducted outside of the U.S., and the findings may not be replicated in future trials at global clinical trial sites in a later stage clinical trial conducted by us or our collaborators. The design of a clinical trial can determine whether its results will support approval of a product and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. We may be unable to design and execute a clinical trial to support marketing approval.
Preclinical and clinical data are often susceptible to varying interpretations and analyses. Many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval for the product candidates. Even if we, or any collaborators, believe that the results of clinical trials for our product candidates warrant marketing approval, the FDA or comparable foreign regulatory authorities may disagree and may not grant marketing approval of our product candidates.
In some instances, there can be significant variability in safety or efficacy results between different clinical trials of the same product candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, changes in and adherence to the dosing regimen and other clinical trial protocols and the rate of dropout among clinical trial participants. If we fail to receive positive results in clinical trials of our product candidates, the development timeline and regulatory approval and commercialization prospects for our most advanced product candidates, and, correspondingly, our business and financial prospects would be negatively impacted.
| -41- |
Our current or future product candidates may cause undesirable side effects or have other properties when used alone or in combination with other approved products or investigational new drugs that could halt their clinical development, prevent their marketing approval, limit their commercial potential or result in significant negative consequences.
Undesirable or clinically unmanageable side effects could occur and cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of marketing approval by the FDA or comparable foreign regulatory authorities. Results of our trials could reveal a high and unacceptable severity and prevalence of side effects or unexpected characteristics.
If unacceptable side effects arise in the development of our product candidates, we, the FDA or comparable foreign regulatory authorities, the Institutional Review Boards, or IRBs, or independent ethics committees at the institutions in which our studies are conducted, or the Data Safety Monitoring Board, or DSMB, could suspend or terminate our clinical trials or the FDA or comparable foreign regulatory authorities could order us to cease clinical trials or deny approval of our product candidates for any or all targeted indications. Treatment-related side effects could also affect patient recruitment or the ability of enrolled subjects to complete the trial, or result in potential product liability claims. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff. We may be required to train medical personnel using our product candidates to understand the side effect profiles for our clinical trials and upon any commercialization of any of our product candidates. Inadequate training in recognizing or managing the potential side effects of our product candidates could result in patient injury or death. Any of these occurrences may prevent us from achieving or maintaining market acceptance of the affected product candidate and may harm our business, financial condition and prospects significantly.
Moreover, clinical trials of our product candidates are conducted in carefully defined sets of patients who have agreed to enter into clinical trials. Consequently, it is possible that our clinical trials may indicate an apparent positive effect of a product candidate that is greater than the actual positive effect, if any, or alternatively fail to identify undesirable side effects. If, following approval of a product candidate, we, or others, discover that the product is less effective than previously believed or causes undesirable side effects that were not previously identified, any of the following consequences could occur:
● regulatory authorities may withdraw their approval of the product or seize the product;
● we, or any collaborators, may need to recall the product, or be required to change the way the product is administered or conduct additional clinical trials;
● additional restrictions may be imposed on the marketing of, or the manufacturing processes for, the particular product;
● we may be subject to fines, injunctions or the imposition of civil or criminal penalties;
● regulatory authorities may require the addition of labeling statements, such as a boxed warning or a contraindication;
● we, or any collaborators, may be required to create a medication guide outlining the risks of the previously unidentified side effects for distribution to patients;
● we, or any collaborators, could be sued and held liable for harm caused to patients;
● the product may become less competitive; and
● our reputation may suffer.
If any of our current or future product candidates fail to demonstrate safety and efficacy in clinical trials or do not gain marketing approval, we will not be able to generate revenue and our business will be harmed. Any of these events could harm our business and operations, and could negatively impact the price of our common stock.
| -42- |
We may not be successful in our efforts to identify or discover additional product candidates.
Although we intend to explore other therapeutic opportunities in addition to the product candidates that we are currently developing, we may fail to identify other product candidates for clinical development for a number of reasons. For example, our research methodology may not be successful in identifying potential product candidates or those we identify may be shown to have harmful side effects or other characteristics that make them unmarketable or unlikely to receive regulatory approval. Additional product candidates will require additional, time-consuming development efforts prior to commercial sale, including preclinical studies, clinical trials and approval by the FDA and/or applicable foreign regulatory authorities. All product candidates are prone to the risks of failure that are inherent in pharmaceutical product development. If we fail to identify and develop additional potential product candidates, we may be unable to grow our business and our results of operations could be materially harmed.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we intend to focus on developing product candidates for specific indications that we identify as most likely to succeed, in terms of both their potential for marketing approval and commercialization. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that may prove to have greater commercial potential.
Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable product candidates. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to the product candidate.
We face potential product liability, and, if successful claims are brought against us, we may incur substantial liability and costs. If the use of our product candidates harms patients, or is perceived to harm patients even when such harm is unrelated to our product candidates, our regulatory approvals could be revoked or otherwise negatively impacted and we could be subject to costly and damaging product liability claims.
The use of our product candidates in clinical trials and the sale of any products for which we obtain marketing approval expose us to the risk of product liability claims. Product liability claims might be brought against us by patients, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our products. There is a risk that our product candidates may induce adverse events. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| ● | the impairment of our business reputation; | |
| ● | the withdrawal of clinical trial participants; | |
| ● | substantial monetary awards to patients or other claimants; | |
| ● | costs due to related litigation; | |
| ● | the distraction of management’s attention from our primary business; | |
| ● | the inability to commercialize our product candidates; and | |
| ● | decreased demand for our product candidates, if approved for commercial sale. |
| -43- |
We intend to acquire product liability insurance coverage in light of our current clinical programs; however, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. We intend to expand our insurance coverage each time we commercialize an additional product; however, we may be unable to obtain product liability insurance on commercially reasonable terms or in adequate amounts. On occasion, large judgments have been awarded in class action lawsuits based on drugs or medical treatments that had unanticipated adverse effects. A successful product liability claim or series of claims brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could adversely affect our results of operations and business.
Patients with the diseases targeted by certain of our product candidates, such as our lead indications in oncology, are often already in severe and advanced stages of disease and have both known and unknown significant pre-existing and potentially life-threatening health risks. During the course of treatment, patients may suffer adverse events, including death, for reasons that may be related to our product candidates. Such events could subject us to costly litigation, require us to pay substantial amounts of money to injured patients, delay, negatively impact or end our opportunity to receive or maintain regulatory approval to market our products, or require us to suspend or abandon our commercialization efforts. Even in a circumstance in which we do not believe that an adverse event is related to our products, the investigation into the circumstance may be time-consuming or inconclusive. These investigations may interrupt our sales efforts, delay our regulatory approval process, or impact and limit the type of regulatory approvals our product candidates receive or maintain. As a result of these factors, a product liability claim, even if successfully defended, could have a material adverse effect on our business, financial condition or results of operations.
We may seek designations for our product candidates with the FDA and other comparable regulatory authorities that are intended to confer benefits such as a faster development process or an accelerated regulatory pathway, but there can be no assurance that we will successfully obtain such designations. In addition, even if one or more of our product candidates are granted such designations, we may not be able to realize the intended benefits of such designations.
The FDA and other comparable regulatory authorities offer certain designations for product candidates that are intended to encourage the research and development of pharmaceutical products addressing conditions with significant unmet medical need. These designations may confer benefits such as additional interaction with regulatory authorities, a potentially accelerated regulatory pathway and priority review. There can be no assurance that we will successfully obtain such designation for any of our other product candidates. In addition, while such designations could expedite the development or approval process, they generally do not change the standards for approval. Even if we obtain such designations for one or more of our product candidates, there can be no assurance that we will realize their intended benefits.
For example, we may seek a Breakthrough Therapy Designation for one or more of our product candidates. A breakthrough therapy is defined as a therapy that is intended, alone or in combination with one or more other therapies, to treat a serious or life-threatening disease or condition, if preliminary clinical evidence indicates that the therapy may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For therapies that have been designated as breakthrough therapies, interaction and communication between the FDA and the sponsor of the trial can help to identify the most efficient path for clinical development while minimizing the number of patients placed in ineffective control regimens. Therapies designated as breakthrough therapies by the FDA are also eligible for accelerated approval. Designation as a breakthrough therapy is within the discretion of the FDA. Accordingly, even if we believe one of our product candidates meets the criteria for designation as a breakthrough therapy, the FDA may disagree and instead determine not to make such designation. In any event, the receipt of a Breakthrough Therapy Designation for a product candidate may not result in a faster development process, review or approval compared to therapies considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even if one or more of our product candidates qualify as breakthrough therapies, the FDA may later decide that such product candidates no longer meet the conditions for qualification.
We may also seek Fast Track Designation for some of our product candidates. If a therapy is intended for the treatment of a serious or life-threatening condition and the therapy demonstrates the potential to address unmet medical needs for this condition, the therapy sponsor may apply for Fast Track Designation. The FDA has broad discretion whether or not to grant this designation, so even if we believe a particular product candidate is eligible for this designation, there can be no assurance that the FDA would decide to grant it. Even if we do receive Fast Track Designation, we may not experience a faster development process, review or approval compared to conventional FDA procedures, and receiving a Fast Track Designation does not provide assurance of ultimate FDA approval. The FDA may withdraw Fast Track Designation if it believes that the designation is no longer supported by data from our clinical development program.
| -44- |
We may seek priority review designation for one or more of our product candidates, but we might not receive such designation, and even if we do, such designation may not lead to a faster regulatory review or approval process.
If the FDA determines that a product candidate offers a treatment for a serious condition and, if approved, the product would provide a significant improvement in safety or effectiveness, the FDA may designate the product candidate for priority review. A priority review designation means that the goal for the FDA to review an application is six months, rather than the standard review period of ten months. We may request priority review for our product candidates. The FDA has broad discretion with respect to whether or not to grant priority review status to a product candidate, so even if we believe a particular product candidate is eligible for such designation or status, in particular if such product candidate has received a Breakthrough Therapy Designation, the FDA may decide not to grant it. Moreover, a priority review designation does not result in expedited development and does not necessarily result in expedited regulatory review or approval process or necessarily confer any advantage with respect to approval compared to conventional FDA procedures. Receiving priority review from the FDA does not guarantee approval within the six-month review cycle or at all.
Obtaining and maintaining marketing approval of our current and future product candidates in one jurisdiction does not mean that we will be successful in obtaining marketing approval of our current and future product candidates in other jurisdictions.
Obtaining and maintaining marketing approval of our current and future product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain marketing approval in any other jurisdiction, while a failure or delay in obtaining marketing approval in one jurisdiction may have a negative effect on the marketing approval process in others. For example, even if the FDA grants marketing approval of a product candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials as clinical studies conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval. We do not have experience in obtaining reimbursement or pricing approvals in international markets.
Obtaining marketing approvals and compliance with regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries outside of the United States. If we fail to comply with the regulatory requirements in international markets and/or receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our product candidates will be harmed.
Risks Related to Commercialization of Our Product Candidates and Other Regulatory Compliance Matters
Even if we complete the necessary preclinical studies and clinical trials, the marketing approval process is expensive, time consuming and uncertain and may prevent us or any collaborators from obtaining approvals for the commercialization of some or all of our product candidates. As a result, we cannot predict when or if, and in which territories, we, or any collaborators, will obtain marketing approval to commercialize a product candidate.
The process of obtaining marketing approvals, both in the United States and abroad, is lengthy, expensive and uncertain. It may take many years, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. Securing marketing approval requires the submission of extensive preclinical and clinical data and supporting information to regulatory authorities for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities. The FDA or other regulatory authorities may determine that our product candidates are not safe and effective, only moderately effective or have undesirable or unintended side effects, toxicities or other characteristics that preclude our obtaining marketing approval or prevent or limit commercial use. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
| -45- |
In addition, changes in marketing approval policies during the development period, changes in or the enactment or promulgation of additional statutes, regulations or guidance or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical, clinical or other studies. Varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of a product candidate. We cannot commercialize a product until the appropriate regulatory authorities have reviewed and approved the product candidate. Even if our product candidates demonstrate safety and efficacy in clinical trials, the regulatory agencies may not complete their review processes in a timely manner, or we may not be able to obtain regulatory approval. Additional delays may result if an FDA Advisory Committee or other regulatory authority recommends non-approval or restrictions on approval. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory agency policy during the period of product development, clinical trials and the review process. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product commercially unviable.
Moreover, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. Under certain circumstances, we may be required to report some of these relationships to the FDA or other regulatory authority. The FDA or other regulatory authority may conclude that a financial relationship between us and a principal investigator has created a conflict of interest or otherwise affected interpretation of the study. The FDA or other regulatory authority may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA or other regulatory authority, as the case may be, and may ultimately lead to the denial of marketing approval of one or more of our product candidates.
In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates. For example, regulatory agencies may approve a product candidate for fewer or more limited indications than requested or may grant approval subject to the performance of post-marketing studies. Regulators may approve a product candidate for a smaller patient population, a different drug formulation or a different manufacturing process, than we are seeking. If we are unable to obtain necessary regulatory approvals, or more limited regulatory approvals than we expect, our business, prospects, financial condition and results of operations may suffer.
Any delay in obtaining or failure to obtain required approvals could negatively impact our ability to generate revenue from the particular product candidate, which likely would result in significant harm to our financial position and adversely impact the price of our common stock.
We currently have no marketing, sales or distribution infrastructure with respect to our product candidates. If we are unable to develop our sales, marketing and distribution capability on our own or through collaborations with marketing partners, we will not be successful in commercializing our product candidates.
We currently have no marketing, sales or distribution capabilities and have limited sales or marketing experience within our organization. If one or more of our product candidates is approved, we intend either to establish a sales and marketing organization with technical expertise and supporting distribution capabilities to commercialize that product candidate, or to outsource this function to a third party. There are risks involved with either establishing our own sales and marketing capabilities and entering into arrangements with third parties to perform these services.
| -46- |
Recruiting and training an internal commercial organization is expensive and time consuming and could delay any product launch. Some or all of these costs may be incurred in advance of any approval of any of our product candidates. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly and our investment would be lost if we cannot retain or reposition our sales and marketing personnel. In addition, we may not be able to hire a sales force in the United States or other target market that is sufficient in size or has adequate expertise in the medical markets that we intend to target.
Factors that may inhibit our efforts to commercialize our product candidates on our own include:
● the inability to recruit, train and retain adequate numbers of effective sales and marketing personnel;
● the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe any future product that we may develop;
● the lack of complementary treatments to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and
● unforeseen costs and expenses associated with creating an independent sales and marketing organization.
If we enter into arrangements with third parties to perform sales, marketing and distribution services, our product revenue or the profitability to us from these revenue streams is likely to be lower than if we were to market and sell any product candidates that we develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell and market our product candidates or may be unable to do so on terms that are favorable to us. We likely will have little control over such third parties and any of them may fail to devote the necessary resources and attention to sell and market our product candidates effectively. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we may not be successful in commercializing our product candidates.
The market opportunities for any current or future product candidate we develop, if and when approved, may be limited to those patients who are ineligible for established therapies or for whom prior therapies have failed, and therefore may be small.
Cancer therapies are sometimes characterized as first-line, second-line, or third-line, and the FDA often approves new therapies initially only for third-line use. When cancer is detected early enough, first-line therapy, usually chemotherapy, hormone therapy, surgery, radiation therapy, immunotherapy or a combination of these, is sometimes adequate to cure the cancer or prolong life without a cure. Second- and third-line therapies are administered to patients when prior therapy is not effective. We may initially seek approval of SON-080 and any other product candidates we develop as a therapy for patients who have received one or more prior treatments. Subsequently, for those products that prove to be sufficiently beneficial, if any, we would expect to seek approval potentially as a first-line therapy, but there is no guarantee that product candidates we develop, even if approved, would be approved for first-line therapy, and, prior to any such approvals, we may have to conduct additional clinical trials.
The number of patients who have the cancers we are targeting may turn out to be lower than expected. Additionally, the potentially addressable patient population for our current programs or future product candidates may be limited, if and when approved. Even if we obtain significant market share for any product candidate, if and when approved, if the potential target populations are small, we may never achieve profitability without obtaining marketing approval for additional indications, including use as first- or second-line therapy.
| -47- |
Even if we receive marketing approval of a product candidate, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our products, if approved.
Any marketing approvals that we receive for any current or future product candidate may be subject to limitations on the approved indicated uses for which the product may be marketed or the conditions of approval, or contain requirements for potentially costly post-market testing and surveillance to monitor the safety and efficacy of the product candidate. The FDA may also require a Risk Evaluation and Mitigation Strategy, or REMS, as a condition of approval of any product candidate, which could include requirements for a medication guide, physician communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. If the FDA or a comparable foreign regulatory authority approves a product candidate, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion, import and export and record keeping for the product candidate will be subject to extensive and ongoing regulatory requirements. These requirements include, among others, prohibitions on the promotion of an approved product for uses not included in the product’s approved labeling, submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with current Good Manufacturing Practice, or cGMP, and Good Clinical Practice, or GCP, for any clinical trials that we conduct post-approval. Later discovery of previously unknown problems with any approved candidate, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
● restrictions on the labeling, distribution, marketing or manufacturing of the product, withdrawal of the product from the market, or product recalls;
● untitled and warning letters, or holds on clinical trials;
● refusal by the FDA to approve pending applications or supplements to approved applications we filed or suspension or revocation of license approvals;
● requirements to conduct post-marketing studies or clinical trials;
● restrictions on coverage by third-party payors;
● fines, restitution or disgorgement of profits or revenues;
● suspension or withdrawal of marketing approvals;
● product seizure or detention, or refusal to permit the import or export of the product; and
● injunctions or the imposition of civil or criminal penalties.
The FDA’s and other regulatory authorities’ policies may change and additional government regulations may be enacted that could prevent, limit or delay marketing approval of a product. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability.
We face significant competition and if our competitors develop and market products that are more effective, safer or less expensive than the product candidates we develop, our commercial opportunities will be negatively impacted.
The life sciences industry is highly competitive. We are currently developing therapeutics that will compete, if approved, with other products and therapies that currently exist, are being developed or will in the future be developed, some of which we may not currently be aware.
| -48- |
We have competitors both in the United States and internationally, including major multinational pharmaceutical companies, established biotechnology companies, specialty pharmaceutical companies, universities and other research institutions. Many of our competitors have significantly greater financial, manufacturing, marketing, product development, technical and human resources than we do. Large pharmaceutical companies, in particular, have extensive experience in clinical testing, obtaining marketing approvals, recruiting patients and manufacturing pharmaceutical products. These companies also have significantly greater research and marketing capabilities than we do and may also have products that have been approved or are in late stages of development, and collaborative arrangements in our target markets with leading companies and research institutions. Established pharmaceutical companies may also invest heavily to accelerate discovery and development of novel compounds or to in-license novel compounds that could make the product candidates that we develop obsolete. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. As a result of all of these factors, our competitors may succeed in obtaining patent protection and/or marketing approval or discovering, developing and commercializing products in our field before we do.
There is a large number of companies developing or marketing treatments for cancer, including many major pharmaceutical and biotechnology companies. These treatments consist both of small molecule drug products, such as traditional chemotherapy, as well as novel immunotherapies. For example, a number of multinational companies as well as large biotechnology companies, including Astellas Pharma Inc., Seattle Genetics, Inc., AstraZeneca, and GlaxoSmithKline plc, are developing programs for the targets that we are exploring for our pipeline programs.
Our commercial opportunities could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe effects, are more convenient, have a broader label, are marketed more effectively, are reimbursed or are less expensive than any products that we may develop. Our competitors also may obtain FDA, EMA or other marketing approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. Even if the product candidate we develop achieve marketing approval, they may be priced at a significant premium over competitive products if any have been approved by then, resulting in reduced competitiveness.
Smaller and other early stage companies may also prove to be significant competitors. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. In addition, the biopharmaceutical industry is characterized by rapid technological change. If we fail to stay at the forefront of technological change, we may be unable to compete effectively. Technological advances or products developed by our competitors may render our product candidates obsolete, less competitive or not economical.
The commercial success of any current or future product candidate will depend upon the degree of market acceptance by physicians, patients, payors and others in the medical community.
We have never commercialized a product, and even if we obtain any regulatory approval for our product candidates, the commercial success of our product candidates will depend in part on the medical community, patients, and payors accepting our product candidates as effective, safe and cost-effective. Any product that we bring to the market may not gain market acceptance by physicians, patients, payors and others in the medical community. Physicians are often reluctant to switch their patients from existing therapies even when new and potentially more effective or convenient treatments enter the market. Further, patients often acclimate to the therapy that they are currently taking and do not want to switch unless their physicians recommend switching products or they are required to switch therapies due to lack of reimbursement for existing therapies.
The degree of market acceptance of these product candidates, if approved for commercial sale, will depend on a number of factors, including:
| ● | the potential efficacy and potential advantages over alternative treatments; | |
| ● | the frequency and severity of any side effects, including any limitations or warnings contained in a product’s approved labeling; | |
| ● | the frequency and severity of any side effects resulting from follow-up requirements for the administration of our product candidates; |
| -49- |
| ● | the relative convenience and ease of administration; | |
| ● | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; | |
| ● | the strength of marketing and distribution support and timing of market introduction of competitive products; | |
| ● | publicity concerning our products or competing products and treatments; and | |
| ● | sufficient third-party insurance coverage and adequate reimbursement. |
Even if a product candidate displays a favorable efficacy and safety profile in preclinical studies and clinical trials, market acceptance of the product, if approved for commercial sale, will not be known until after it is launched. Our efforts to educate the medical community and payors on the benefits of our product candidates may require significant resources and may never be successful. Such efforts to educate the marketplace may require more resources than are required by the conventional technologies marketed by our competitors, particularly due to the novelty of our Sonnet approach. If these products do not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable.
If the market opportunities for our product candidates are smaller than we believe they are, our product revenues may be adversely affected and our business may suffer.
We currently focus our research and product development on treatments for oncology indications and our product FHAB candidates are designed to target solid tumors. Our understanding of both the number of people who have these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our product candidates, are based on estimates. These estimates may prove to be incorrect and new studies may reduce the estimated incidence or prevalence of these diseases. Patient identification efforts also influence the ability to address a patient population. If efforts in patient identification are unsuccessful or less impactful than anticipated, we may not address the entirety of the opportunity we are seeking.
The insurance coverage and reimbursement status of newly-approved products is uncertain. Failure to obtain or maintain adequate coverage and reimbursement for any of our product candidates, if approved, could limit our ability to market those products and decrease our ability to generate revenue.
We expect the cost of our product candidates to be substantial, when and if they achieve market approval. The availability and extent of reimbursement by governmental and private payors is essential for most patients to be able to afford expensive treatments. Sales of our product candidates will depend substantially, both domestically and abroad, on the extent to which the costs of our product candidates will be paid by private payors, such as private health coverage insurers, health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by government health care programs, such as Medicare and Medicaid. We may not be able to provide data sufficient to gain acceptance with respect to coverage and reimbursement. If reimbursement is not available, or is available only at limited levels, we may not be able to successfully commercialize our product candidates, even if approved. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, the principal decisions about coverage and reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services, or CMS, an agency within the U.S. Department of Health and Human Services, as the CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private payors tend to follow CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to coverage and reimbursement for novel products such as ours, as there is no body of established practices and precedents for these new products. Coverage and reimbursement by a third-party payor may depend upon a number of factors, including the third-party payor’s determination that use of a product is: (1) a covered benefit under its health plan; (2) safe, effective and medically necessary; (3) appropriate for the specific patient; (4) cost-effective; and (5) neither experimental nor investigational. In the United States, no uniform policy of coverage and reimbursement for products exists among third-party payors. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. Even if we obtain coverage for a given product, the resulting reimbursement payment rates might not be adequate for us to achieve or sustain profitability or may require co-payments that patients find unacceptably high. Third-party payors may limit coverage to specific drug products on an approved list, also known as a formulary, which might not include all of the approved drugs for a particular indication.
| -50- |
Additionally, third-party payors may not cover, or provide adequate reimbursement for, long-term follow-up evaluations required following the use of product candidates. Patients are unlikely to use our product candidates unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our product candidates. Because our product candidates may have a higher cost of goods than conventional therapies, and may require long-term follow-up evaluations, the risk that coverage and reimbursement rates may be inadequate for us to achieve profitability may be greater. There is significant uncertainty related to insurance coverage and reimbursement of newly approved products. It is difficult to predict at this time what third-party payors will decide with respect to the coverage and reimbursement for our product candidates.
Moreover, increasing efforts by governmental and third-party payors in the United States and abroad to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for our product candidates. There has been increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. We expect to experience pricing pressures in connection with the sale of any of our product candidates due to the trend toward managed healthcare, the increasing influence of health maintenance organizations, cost containment initiatives and additional legislative changes.
Outside the United States, certain countries, including a number of member states of the European Union, set prices and reimbursement for pharmaceutical products, or medicinal products, as they are commonly referred to in the European Union. These countries have broad discretion in setting prices and we cannot be sure that such prices and reimbursement will be acceptable to us or our collaborators. If the regulatory authorities in these jurisdictions set prices or reimbursement levels that are not commercially attractive for us or our collaborators, our revenues from sales by us or our collaborators, and the potential profitability of our drug products, in those countries would be negatively affected. An increasing number of countries are taking initiatives to attempt to reduce large budget deficits by focusing cost-cutting efforts on pharmaceuticals for their state-run health care systems. These international price control efforts have impacted all regions of the world, but have been most drastic in the European Union. Additionally, some countries require approval of the sale price of a product before it can be lawfully marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. To obtain reimbursement or pricing approval in some countries, we, or any collaborators, may be required to conduct a clinical trial that compares the cost-effectiveness of our product to other available therapies. As a result, we might obtain marketing approval for a product in a particular country, but then may experience delays in the reimbursement approval of our product or be subject to price regulations that would delay our commercial launch of the product, possibly for lengthy time periods, which could negatively impact the revenues we are able to generate from the sale of the product in that particular country.
Moreover, efforts by governments and payors, in the United States and abroad, to cap or reduce healthcare costs may cause such organizations to limit both coverage and level of reimbursement for new products approved and, as a result, they may not cover or provide adequate reimbursement for our product candidates. There has been increasing legislative and enforcement interest in the United States with respect to specialty drug practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. We expect to experience pricing pressures in connection with the sale of any of our product candidates, due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be harmed.
| -51- |
If the FDA or comparable foreign regulatory authorities approve generic versions of any of our product candidates that receive marketing approval, or such authorities do not grant such products appropriate periods of data exclusivity before approving generic versions of such products, the sales of such products could be adversely affected.
In the United States, manufacturers may seek approval of biosimilar versions of biologics approved by the FDA under a BLA through submission of abbreviated biologic license applications, or ABLAs. In support of an ABLA, a biosimilar manufacturer generally must show that its product is similar to the original biologic product. Biosimilar products may be less costly to bring to market than the original biologic and companies that produce biosimilar products are sometimes able to offer them at lower prices. Thus, following the introduction of a biosimilar product, a significant percentage of the sales of the original biologic may be lost to the biosimilar product, and the price of the original biologic product may be lowered.
The FDA may not accept for review or approve an ABLA for a biosimilar product until any applicable period of non-patent exclusivity for the original biologic has expired. The Public Health Service (PHS) Act provides a period of twelve years of non-patent exclusivity for a biologic approved under a BLA.
Competition that our products may face from biosimilar versions of our products could negatively impact our future revenue, profitability and cash flows and substantially limit our ability to obtain a return on our investments in those product candidates.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws health information privacy and security laws, and other health care laws and regulations. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
If we obtain FDA approval for any of our product candidates and begin commercializing those products in the United States, our operations will be directly, or indirectly through our prescribers, customers and purchasers, subject to various federal and state fraud and abuse laws and regulations, including, without limitation, the federal Health Care Program Anti-Kickback Statute, or Anti-Kickback Statute, the federal civil and criminal False Claims Act and Physician Payments Sunshine Act and regulations. These laws will impact, among other things, our proposed sales, marketing and educational programs. In addition, we may be subject to patient privacy laws by both the federal government and the states in which we conduct our business. The laws that will affect our operations include, but are not limited to:
| ● | the Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, either the referral of an individual, or the purchase, lease, order, arrangement, or recommendation of any good, facility, item or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. “Remuneration” has been interpreted broadly to include anything of value. A person or entity does not need to have actual knowledge of the Anti-Kickback Statute or specific intent to violate it to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act, or FCA, or federal civil money penalties. The Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers, and formulary managers on the other. There are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution; |
| -52- |
| ● | the federal civil and criminal false claims laws and civil monetary penalty laws, including the FCA, which impose criminal and civil penalties against individuals or entities for, among other things: knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent; knowingly making, using or causing to be made or used, a false statement of record material to a false or fraudulent claim or obligation to pay or transmit money or property to the federal government. Manufacturers can be held liable under the FCA even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims. The FCA also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the FCA and to share in any monetary recovery; | |
| ● | the beneficiary inducement provisions of the CMP Law, which prohibits, among other things, the offering or giving of remuneration, which includes, without limitation, any transfer of items or services for free or for less than fair market value (with limited exceptions), to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of items or services reimbursable by a federal or state governmental program; | |
| ● | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit a person from knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false, fictitious, or fraudulent statements or representations in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters; similar to the Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; | |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, and their respective implementing regulations, which impose requirements on certain healthcare providers, health plans, and healthcare clearinghouses, known as covered entities, as well as their respective business associates, individuals and entities that perform services on their behalf that involve the use or disclosure of individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information; | |
| ● | the U.S. federal transparency requirements under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, ACA, including the provision commonly referred to as the Physician Payments Sunshine Act, which requires applicable manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to CMS information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by the physicians described above and their immediate family members. Beginning in 2022, applicable manufacturers also will be required to report information regarding payments and transfers of value provided to physician assistants, nurse practitioners, clinical nurse specialists, certified nurse anesthetists, and certified nurse-midwives; | |
| ● | federal government price reporting laws, which require us to calculate and report complex pricing metrics in an accurate and timely manner to government programs; and | |
| ● | federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers. |
| -53- |
Additionally, we are subject to state and foreign equivalents of each of the healthcare laws and regulations described above, among others, some of which may be broader in scope and may apply regardless of the payer. Many U.S. states have adopted laws similar to the Anti-Kickback Statute and False Claims Act, and may apply to our business practices, including, but not limited to, research, distribution, sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental payors, including private insurers. In addition, some states have passed laws that require pharmaceutical companies to comply with the April 2003 Office of Inspector General Compliance Program Guidance for Pharmaceutical Manufacturers and/or the Pharmaceutical Research and Manufacturers of America’s Code on Interactions with Healthcare Professionals. Several states also impose other marketing restrictions or require pharmaceutical companies to make marketing or price disclosures to the state. There are ambiguities as to what is required to comply with these state requirements and if we fail to comply with an applicable state law requirement we could be subject to penalties. Finally, there are state and foreign laws governing the privacy and security of health information, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Because of the breadth of these laws and the narrowness of the statutory exceptions and regulatory safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. Law enforcement authorities are increasingly focused on enforcing fraud and abuse laws, and it is possible that some of our practices may be challenged under these laws. Efforts to ensure that our current and future business arrangements with third parties, and our business generally, will comply with applicable healthcare laws and regulations will involve substantial costs. If our operations, including our arrangements with physicians and other healthcare providers, some of whom receive stock options as compensation for services provided, are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, contractual damages, reputational harm, diminished profits and future earnings, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs (such as Medicare and Medicaid), additional reporting requirements and/or oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and individual imprisonment, any of which could adversely affect our ability to operate our business and our financial results. Any action for violation of these laws, even if successfully defended, could cause a pharmaceutical manufacturer to incur significant legal expenses and divert management’s attention from the operation of the business. Prohibitions or restrictions on sales or withdrawal of future marketed products could materially affect business in an adverse way.
Healthcare legislative reform measures and constraints on national budget social security systems may have a material adverse effect on our business and results of operations.
Payors, whether domestic or foreign, or governmental or private, are developing increasingly sophisticated methods of controlling healthcare costs and those methods are not always specifically adapted for new technologies such as those we are developing. In both the United States and certain foreign jurisdictions, there have been a number of legislative and regulatory changes to the health care system that could impact our ability to sell our products profitably. In particular, in the United States, the ACA was enacted in 2010 which, among other things, subjects biologic products to potential competition by lower-cost biosimilars; addresses a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected; increases the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program; extends the Medicaid Drug Rebate program to utilization of prescriptions of individuals enrolled in Medicaid managed care organizations; subjects manufacturers to new annual fees and taxes for certain branded prescription drugs; and provides incentives to programs that increase the federal government’s comparative effectiveness research.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the ACA, as well as recent efforts by the current administration to repeal or replace certain aspects of the ACA. Further, since January 2017, President Trump has signed two Executive Orders and other directives designed to delay the implementation of certain provision of the ACA or otherwise circumvent some of the requirements for health insurance mandated by the ACA. In addition, CMS recently issued a final rule that will give states greater flexibility, starting in 2020, in setting benchmarks for insurers in the individual and small group marketplaces, which may have the effect of relaxing the essential health benefits required under the ACA for plans sold through such marketplaces.
| -54- |
Concurrently, Congress has considered legislation that would repeal and replace all or part of the ACA. While Congress has not passed comprehensive repeal legislation, two bills affecting the implementation of certain taxes under the ACA have been signed into law. The Tax Cuts and Jobs Act of 2017, or TCJA, includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” Additionally, on January 22, 2018, President Trump signed a continuing resolution on appropriations for fiscal year 2018 that delayed the implementation of certain ACA-mandated fees, including the so-called “Cadillac” tax on certain high cost employer-sponsored insurance plans, the annual fee imposed on certain high cost employer-sponsored insurance plans, the annual fee imposed on certain health insurance providers based on market share, and the medical device exercise tax on non-exempt medical devices. Further, the Bipartisan Budget Act of 2018, or BBA, among other things, amends the ACA, effective January 1, 2019, to increase from 50 percent to 70 percent the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D and to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole.” More recently, in July 2018, CMS published a final rule permitting further collections and payments to and from certain ACA qualified health plans and health insurance issuers under the ACA risk adjustment program in response to the outcome of federal district court litigation regarding the method CMS uses to determine this risk adjustment. Congress also could consider additional legislation to repeal or replace other elements of the ACA. Thus, the full impact of the ACA, any law repealing or replacing elements of it, and the political uncertainty surrounding any repeal or replacement legislation on our business remains unclear.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.5 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013, and due to subsequent legislative amendments, including the BBA, will remain in effect through 2027 unless additional Congressional action is taken. In January 2013, the American Taxpayer Relief Act of 2012, was signed into law, which, among other things, further reduced Medicare payments to several providers, including hospitals and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Also, there has been heightened governmental scrutiny recently over the manner in which drug manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. For example, the current administration released a “Blueprint” to lower drug prices and reduce out of pocket costs of drugs that contains additional proposals to increase manufacturer competition, increase the negotiating power of certain federal healthcare programs, incentivize manufacturers to lower the list price of their products and reduce the out of pocket costs of drug products paid by consumers. For example, in November 2018, CMS issued a proposed rule for comment that would, among other things, provide Medicare prescription drug plans under Part D more transparency in pricing and greater flexibility to negotiate discounts for, and in certain circumstances exclude, drugs in the six “protected” formulary classes and allow Medicare Advantage plans to use certain drug management tools such as step therapy for physician-administered drugs. Although a number of these, and other proposed measures will require authorization through additional legislation to become effective, Congress and the Trump administration have each indicated that it will continue to seek new legislative and/or administrative measures to control drug costs.
There have been, and likely will continue to be, legislative and regulatory proposals at the foreign, federal and state levels directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of these governments and other payors to contain or reduce costs of healthcare and/or impose price controls may adversely affect:
| ● | the demand for our product candidates, if we obtain regulatory approval; | |
| ● | our ability to set a price that we believe is fair for our products; | |
| ● | our ability to generate revenue and achieve or maintain profitability; | |
| ● | the level of taxes that we are required to pay; and | |
| ● | the availability of capital. |
| -55- |
Any denial in coverage or reduction in reimbursement from Medicare or other government programs may result in a similar denial or reduction in payments from private payors, which may adversely affect our future profitability.
We are subject to the the U.S. Foreign Corrupt Practices Act of 1977, as amended, or the FCPA, and other anti-corruption laws, as well as export control laws, import and customs laws, trade and economic sanctions laws and other laws governing our operations.
Our operations are subject to anti-corruption laws, including the FCPA, the U.S. domestic bribery statute contained in 18 §201, the U.S. Travel Act, and other anti-corruption laws that apply in countries where we do business. The Bribery Act, the FCPA and these other laws generally prohibit us and our employees and intermediaries from authorizing, promising, offering, or providing, directly or indirectly, improper or prohibited payments, or anything else of value, to government officials or other persons to obtain or retain business or gain some other business advantage. Under the Bribery Act, we may also be liable for failing to prevent a person associated with us from committing a bribery offense. We and our commercial partners operate in a number of jurisdictions that pose a high risk of potential Bribery Act or FCPA violations, and we participate in collaborations and relationships with third parties whose corrupt or illegal activities could potentially subject us to liability under the Bribery Act, FCPA or local anti-corruption laws, even if we do not explicitly authorize or have actual knowledge of such activities. In addition, we cannot predict the nature, scope or effect of future regulatory requirements to which our international operations might be subject or the manner in which existing laws might be administered or interpreted.
We are also subject to other laws and regulations governing our international operations, including regulations administered by the governments of the United Kingdom and the United States, and authorities in the European Union, including applicable export control regulations, economic sanctions and embargoes on certain countries and persons, anti-money laundering laws, import and customs requirements and currency exchange regulations, collectively referred to as the Trade Control laws.
There is no assurance that we will be completely effective in ensuring our compliance with all applicable anti-corruption laws, including the Bribery Act, the FCPA or other legal requirements, including Trade Control laws. If we are not in compliance with the Bribery Act, the FCPA and other anti-corruption laws or Trade Control laws, we may be subject to criminal and civil penalties, disgorgement and other sanctions and remedial measures, and legal expenses, which could have an adverse impact on our business, financial condition, results of operations and liquidity. Likewise, any investigation of any potential violations of the Bribery Act, the FCPA, other anti-corruption laws or Trade Control laws by United Kingdom, United States or other authorities could also have an adverse impact on our reputation, our business, results of operations and financial condition.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties. Furthermore, environmental laws and regulations are complex, change frequently and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
| -56- |
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials or other work-related injuries, this insurance may not provide adequate coverage against potential liabilities. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions or liabilities, which could materially adversely affect our business, financial condition, results of operations and prospects.
Risks Related to Our International Operations
As one of our subsidiaries, Relief, is based outside of the United States, we are subject to economic, political, regulatory and other risks associated with international operations.
As Relief is based in the Switzerland, our business is subject to risks associated with conducting business outside of the United States. Many of our suppliers and clinical trial relationships are located outside the United States. Accordingly, our future results could be harmed by a variety of factors, including:
| ● | economic weakness, including inflation, or political instability in particular non-U.S. economies and markets; | |
| ● | differing and changing regulatory requirements for product approvals; | |
| ● | differing jurisdictions could present different issues for securing, maintaining or obtaining freedom to operate in such jurisdictions; | |
| ● | potentially reduced protection for intellectual property rights; | |
| ● | difficulties in compliance with different, complex and changing laws, regulations and court systems of multiple jurisdictions and compliance with a wide variety of foreign laws, treaties and regulations; | |
| ● | changes in non-U.S. regulations and customs, tariffs and trade barriers; | |
| ● | changes in non-U.S. currency exchange rates of the pound sterling, U.S. dollar, euro and currency controls; | |
| ● | trade protection measures, import or export licensing requirements or other restrictive actions by governments; | |
| ● | differing reimbursement regimes and price controls in certain non-U.S. markets; | |
| ● | negative consequences from changes in tax laws; | |
| ● | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad, including, for example, the variable tax treatment in different jurisdictions of options granted under our share option schemes or equity incentive plans; | |
| ● | workforce uncertainty in countries where labor unrest is more common than in the United States; | |
| ● | litigation or administrative actions resulting from claims against us by current or former employees or consultants individually or as part of class actions, including claims of wrongful terminations, discrimination, misclassification or other violations of labor law or other alleged conduct; | |
| ● | difficulties associated with staffing and managing international operations, including differing labor relations; | |
| ● | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and | |
| ● | business interruptions resulting from geo-political actions, including war and terrorism, or natural disasters including earthquakes, typhoons, floods and fires. |
| -57- |
European data collection is governed by restrictive regulations governing the use, processing, and cross-border transfer of personal information.
The collection and use of personal health data in the European Union is was governed by the provisions of the Data Protection Directive, and which, as of May 25, 2018, has been superseded by the GDPR. These directives impose several requirements relating to the consent of the individuals to whom the personal data relates, the information provided to the individuals, notification of data processing obligations to the competent national data protection authorities and the security and confidentiality of the personal data. The Data Protection Directive and GDPR also impose strict rules on the transfer of personal data out of the European Union to the United States. Failure to comply with the requirements of the Data Protection Directive, the GDPR, and the related national data protection laws of the European Union Member States may result in fines and other administrative penalties. While the Data Protection Directive did not apply to organizations based outside the EU, the GDPR has expanded its reach to include any business, regardless of its location, that provides goods or services to residents in the EU. This expansion would incorporate any potential clinical trial activities in EU member states. The GDPR imposes strict requirements on controllers and processors of personal data, including special protections for “sensitive information” which includes health and genetic information of data subjects residing in the EU. GDPR grants individuals the opportunity to object to the processing of their personal information, allows them to request deletion of personal information in certain circumstances, and provides the individual with an express right to seek legal remedies in the event the individual believes his or her rights have been violated. Further, the GDPR imposes strict rules on the transfer of personal data out of the European Union to the United States or other regions that have not been deemed to offer “adequate” privacy protections. Failure to comply with the requirements of the GDPR and the related national data protection laws of the European Union Member States, which may deviate slightly from the GDPR, may result in fines of up to 4% of global revenues, or € 20,000,000, whichever is greater. As a result of the implementation of the GDPR, we may be required to put in place additional mechanisms ensuring compliance with the new data protection rules.
Risks Related to Our Dependence on Third Parties
For certain product candidates, we may depend on development and commercialization collaborators to develop and conduct clinical trials with, obtain regulatory approvals for, and if approved, market and sell product candidates. If such collaborators fail to perform as expected, the potential for us to generate future revenue from such product candidates would be significantly reduced and our business would be harmed.
For certain products candidates, we depend, or will depend, on our development and commercial collaborators to develop, conduct clinical trials of, and, if approved, commercialize product candidates.
Our current collaborations and any future collaborations that we enter into are subject to numerous risks, including:
| ● | collaborators have significant discretion in determining the efforts and resources that they will apply to the collaborations; | |
| ● | collaborators may not perform their obligations as expected or fail to fulfill their responsibilities in a timely manner, or at all; |
| -58- |
| ● | collaborators may not pursue development and commercialization of any product candidates that achieve regulatory approval or may elect not to continue or renew development or commercialization programs based on preclinical studies or clinical trial results, changes in the collaborators’ strategic focus or available funding or external factors, such as an acquisition, that divert resources or create competing priorities; | |
| ● | collaborators may delay preclinical studies or clinical trials, provide insufficient funding for clinical trials, stop a preclinical study or clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; | |
| ● | we may not have access to, or may be restricted from disclosing, certain information regarding product candidates being developed or commercialized under a collaboration and, consequently, may have limited ability to inform our shareholders about the status of such product candidates; | |
| ● | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; | |
| ● | The collaborations may not result in product candidates to develop and/or preclinical studies or clinical trials conducted as part of the collaborations may not be successful; | |
| ● | product candidates developed with collaborators may be viewed by our collaborators as competitive with their own product candidates or products, which may cause collaborators to stop commercialization of our product candidates; | |
| ● | a collaborator with marketing and distribution rights to one or more of our product candidates that achieve regulatory approval may not commit sufficient resources to the marketing and distribution of any such product candidate; and | |
| ● | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation. |
In addition, certain collaboration and commercialization agreements provide our collaborators with rights to terminate such agreements, which rights may or may not be subject to conditions, and which rights, if exercised, would adversely affect our product development efforts and could make it difficult for us to attract new collaborators. In that event, we would likely be required to limit the size and scope of efforts for the development and commercialization of such product candidates or products; we would likely be required to seek additional financing to fund further development or identify alternative strategic collaborations; our potential to generate future revenue from royalties and milestone payments from such product candidates or products would be significantly reduced, delayed or eliminated; and it could have an adverse effect on our business and future growth prospects. Our rights to recover tangible and intangible assets and intellectual property rights needed to advance a product candidate or product after termination of a collaboration may be limited by contract, and we may not be able to advance a program post- termination.
If conflicts arise with our development and commercialization collaborators or licensors, they may act in their own self-interest, which may be adverse to the interests of our company.
We may in the future experience disagreements with our development and commercialization collaborators or licensors. Conflicts may arise in our collaboration and license arrangements with third parties due to one or more of the following:
| ● | disputes with respect to milestone, royalty and other payments that are believed due under the applicable agreements; | |
| ● | disagreements with respect to the ownership of intellectual property rights or scope of licenses; |
| -59- |
| ● | disagreements with respect to the scope of any reporting obligations; | |
| ● | unwillingness on the part of a collaborator to keep us informed regarding the progress of its development and commercialization activities, or to permit public disclosure of these activities; and | |
| ● | disputes with respect to a collaborator’s or our development or commercialization efforts with respect to our products and product candidates. |
Conflicts with our development and commercialization collaborators or licensors could materially adversely affect our business, financial condition or results of operations and future growth prospects.
We will rely on third parties, including independent clinical investigators and CROs, to conduct and sponsor some of the clinical trials of our product candidates. Any failure by a third party to meet its obligations with respect to the clinical development of our product candidates may delay or impair our ability to obtain regulatory approval for our product candidates.
We will be relying upon and plan to continue to rely upon third parties, including independent clinical investigators, academic partners, regulatory affairs consultants and third-party CROs, to conduct our preclinical studies and clinical trials, including in some instances sponsoring such clinical trials, and to engage with regulatory authorities and monitor and manage data for our ongoing preclinical and clinical programs. Given the breadth of clinical therapeutic areas for which we believe our product candidates may have utility, we intend to continue to rely on external service providers rather than build internal regulatory expertise.
Any of these third parties may terminate their engagements with us under certain circumstances. We may not be able to enter into alternative arrangements or do so on commercially reasonable terms. In addition, there is a natural transition period when a new contract research organization begins work. As a result, delays would likely occur, which could negatively impact our ability to meet our expected clinical development timelines and harm our business, financial condition and prospects.
We remain responsible for ensuring that each of our preclinical studies and clinical trials is conducted in accordance with the applicable protocol and legal, regulatory and scientific standards, and our reliance on these third parties does not relieve us of our regulatory responsibilities. We and our third-party contractors and CROs are required to comply with GCP requirements, which are regulations and guidelines enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area, or EEA, and comparable foreign regulatory authorities for all of our products in clinical development. Regulatory authorities enforce these GCP requirements through periodic inspections of trial sponsors, principal investigators and trial sites. If we fail to exercise adequate oversight over any of our academic partners or CROs or if we or any of our academic partners or CROs do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements, or for any other reasons, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, the EMA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon a regulatory inspection of us, our academic partners or our CROs or other third parties performing services in connection with our clinical trials, such regulatory authority will determine that any of our clinical trials complies with GCP regulations. In addition, our clinical trials must be conducted with product produced under applicable CGMP regulations. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process.
Furthermore, the third parties conducting clinical trials on our behalf are not our employees, and except for remedies available to us under our agreements with such contractors, we cannot control whether or not they devote sufficient time, skill and resources to our ongoing development programs. These contractors may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials or other drug development activities, which could impede their ability to devote appropriate time to our clinical programs. If these third parties, including clinical investigators, do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we may not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates. If that occurs, we will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates.
| -60- |
In addition, with respect to investigator-sponsored trials that may be conducted, we would not control the design or conduct of these trials, and it is possible that the FDA or EMA will not view these investigator-sponsored trials as providing adequate support for future clinical trials or market approval, whether controlled by us or third parties, for any one or more reasons, including elements of the design or execution of the trials or safety concerns or other trial results. We expect that such arrangements will provide us certain information rights with respect to the investigator-sponsored trials, including access to and the ability to use and reference the data, including for our own regulatory submissions, resulting from the investigator-sponsored trials. However, we would not have control over the timing and reporting of the data from investigator-sponsored trials, nor would we own the data from the investigator- sponsored trials. If we are unable to confirm or replicate the results from the investigator-sponsored trials or if negative results are obtained, we would likely be further delayed or prevented from advancing further clinical development. Further, if investigators or institutions breach their obligations with respect to the clinical development of our product candidates, or if the data proves to be inadequate compared to the firsthand knowledge we might have gained had the investigator-sponsored trials been sponsored and conducted by us, then our ability to design and conduct any future clinical trials ourselves may be adversely affected. Additionally, the FDA or EMA may disagree with the sufficiency of our right of reference to the preclinical, manufacturing or clinical data generated by these investigator-sponsored trials, or our interpretation of preclinical, manufacturing or clinical data from these investigator-sponsored trials. If so, the FDA or EMA may require us to obtain and submit additional preclinical, manufacturing, or clinical data.
We intend to rely on third parties to manufacture product candidates, which increases the risk that we will not have sufficient quantities of such product candidates or products or such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts.
We do not own or operate manufacturing facilities for the production of clinical or commercial supplies of the product candidates that we are developing or evaluating in our development programs. We have limited personnel with experience in drug manufacturing and lack the resources and the capabilities to manufacture any of our product candidates on a clinical or commercial scale. We rely on third parties for supply of our product candidates, and our strategy is to outsource all manufacturing of our product candidates and products to third parties.
In order to conduct clinical trials of product candidates, we will need to have them manufactured in potentially large quantities. Our third- party manufacturers may be unable to successfully increase the manufacturing capacity for any of our product candidates in a timely or cost- effective manner, or at all. In addition, quality issues may arise during scale-up activities and at any other time. For example, ongoing data on the stability of our product candidates may shorten the expiry of our product candidates and lead to clinical trial material supply shortages, and potentially clinical trial delays. If these third-party manufacturers are unable to successfully scale up the manufacture of our product candidates in sufficient quality and quantity, the development, testing and clinical trials of that product candidate may be delayed or infeasible, and regulatory approval or commercial launch of that product candidate may be delayed or not obtained, which could significantly harm our business.
Our use of new third-party manufacturers increases the risk of delays in production or insufficient supplies of our product candidates as we transfer our manufacturing technology to these manufacturers and as they gain experience manufacturing our product candidates. Even after a third-party manufacturer has gained significant experience in manufacturing our product candidates or even if we believe we have succeeded in optimizing the manufacturing process, there can be no assurance that such manufacturer will produce sufficient quantities of our product candidates in a timely manner or continuously over time, or at all.
We may be delayed if we need to change the manufacturing process used by a third party. Further, if we change an approved manufacturing process, then we may be delayed if the FDA or a comparable foreign authority needs to review the new manufacturing process before it may be used.
| -61- |
We operate an outsourced model for the manufacture of our product candidates, and contract with good manufacturing practice, or GMP, licensed pharmaceutical contract development and manufacturing organizations. While we have engaged several third-party vendors to provide clinical and non-clinical supplies and fill-finish services, we do not currently have any agreements with third-party manufacturers for long-term commercial supplies. In the future, we may be unable to enter into agreements with third-party manufacturers for commercial supplies of any product candidate that we develop, or may be unable to do so on acceptable terms. Even if we are able to establish and maintain arrangements with third-party manufacturers, reliance on third- party manufacturers entails risks, including:
| ● | reliance on third-parties for manufacturing process development, regulatory compliance and quality assurance; | |
| ● | limitations on supply availability resulting from capacity and scheduling constraints of third-parties; | |
| ● | the possible breach of manufacturing agreements by third-parties because of factors beyond our control; and | |
| ● | the possible termination or non-renewal of the manufacturing agreements by the third-party, at a time that is costly or inconvenient to us. |
Third-party manufacturers may not be able to comply with cGMP requirements or similar regulatory requirements outside the United States. Our failure, or the failure of our third-party manufacturers, to comply with applicable requirements could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or products, operating restrictions and/or criminal prosecutions, any of which could significantly and adversely affect supplies of our product candidates. In addition, some of the product candidates we intend to develop, including SON-080, use toxins or other substances that can be produced only in specialized facilities with specific authorizations and permits, and there can be no guarantee that we or our manufacturers can maintain such authorizations and permits. These specialized requirements may also limit the number of potential manufacturers that we can engage to produce our product candidates, and impair any efforts to transition to replacement manufacturers.
Our future product candidates and any products that we may develop may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP requirements that might be capable of manufacturing for us.
If the third parties that we engage to supply any materials or manufacture product for our preclinical tests and clinical trials should cease to continue to do so for any reason, we likely would experience delays in advancing these tests and trials while we identify and qualify replacement suppliers or manufacturers and we may be unable to obtain replacement supplies on terms that are favorable to us. In addition, if we are not able to obtain adequate supplies of our product candidates or the substances used to manufacture them, it will be more difficult for us to develop our product candidates and compete effectively.
Our current and anticipated future dependence upon others for the manufacture of our product candidates may adversely affect our future profit margins and our ability to develop product candidates and commercialize any products that receive marketing approval on a timely and competitive basis.
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we rely on third parties to manufacture our product candidates, and because we collaborate with various organizations and academic institutions on the development of our product candidates, we must, at times, share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, such as trade secrets.
| -62- |
Despite the contractual provisions employed when working with third parties, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure would impair our competitive position and may have a material adverse effect on our business.
In addition, these agreements typically restrict the ability of our collaborators, advisors, employees and consultants to publish data potentially relating to our trade secrets. Our academic collaborators typically have rights to publish data, provided that we are notified in advance and may delay publication for a specified time in order to secure our intellectual property rights arising from the collaboration. In other cases, publication rights are controlled exclusively by us, although in some cases we may share these rights with other parties. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of these agreements, independent development or publication of information including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication. A competitor’s discovery of our trade secrets would impair our competitive position and have an adverse impact on our business.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain patent and other intellectual property protection for our products and product candidates, or if the scope of the patent and other intellectual property protection obtained is not sufficiently broad, our competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize our products and product candidates may be adversely affected.
Our ability to compete effectively will depend, in part, on our ability to maintain the proprietary nature of our technology and manufacturing processes. We rely on research, manufacturing and other know-how, patents, trade secrets, license agreements and contractual provisions to establish our intellectual property rights and protect our products and product candidates. These legal means, however, afford only limited protection and may not adequately protect our rights. As of December 14, 2022, our intellectual property portfolio includes 14 total pending applications and patents, inclusive of 1 issued patent in the U.S., 1 Decision to Grant in Japan and 10 PCT applications within the 5007 patent family – also, 4 pending provisional applications covering formulations, manufacturing processes and methods of use.
In certain situations and as considered appropriate, we have sought, and we intend to continue to seek to protect our proprietary position by filing patent applications in the United States and, in at least some cases, one or more countries outside the United States relating to current and future products and product candidates that are important to our business. However, we cannot predict whether the patent applications currently being pursued will issue as patents, or whether the claims of any resulting patents will provide us with a competitive advantage or whether we will be able to successfully pursue patent applications in the future relating to our current or future products and product candidates. Moreover, the patent application and approval process is expensive and time-consuming. We may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. Furthermore, we, or any future partners, collaborators, or licensees, may fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. Therefore, we may miss potential opportunities to seek additional patent protection. It is possible that defects of form in the preparation or filing of patent applications may exist, or may arise in the future, for example with respect to proper priority claims, inventorship, claim scope, or requests for patent term adjustments. If we fail to establish, maintain or protect such patents and other intellectual property rights, such rights may be reduced or eliminated. If there are material defects in the form, preparation, prosecution or enforcement of our patents or patent applications, such patents may be invalid and/or unenforceable, and such applications may never result in valid, enforceable patents.
Even if they are unchallenged, our patents and patent applications, if issued, may not provide us with any meaningful protection or prevent competitors from designing around our patent claims by developing similar or alternative technologies or therapeutics in a non-infringing manner. For example, a third party may develop a competitive therapy that provides benefits similar to one or more of our product candidates but that falls outside the scope of our patent protection. If the patent protection provided by the patents and patent applications we hold or pursue with respect to our product candidates is not sufficiently broad to impede such competition, our ability to successfully commercialize our product candidates could be negatively affected.
| -63- |
As discussed under the heading “BUSINESS”, our PCT patent application having international patent application number PCT/US2018/00085 received an application filing date of February 20, 2018, which is four days after the one year anniversary of the filing date of U.S. provisional patent applications U.S. 62/459,975 and U.S. 62/459,981 to which the PCT patent application claims a priority benefit due to a computer issue at the PCT receiving office. Despite the restoration of the priority benefit to the filing date of U.S. provisional patent applications (U.S. 62/459,975 and U.S. 62/459,981) by the PCT, some countries in which national stage patent applications were filed from this PCT patent application did not accept this restoration including Canada and China, and the restoration procedure is pending in Brazil. In the event that priority is not restored, prior art may be available to these patent applications that may otherwise not be available to other patent applications filed from PCT/US2018/00085. This could affect the scope or breadth of the patent claims we are pursuing in Brazil, Canada, and China, or could result in no ability to receive patents in these countries.
Other parties, many of whom have substantially greater resources and have made significant investments in competing technologies, have developed or may develop technologies that may be related or competitive with our approach, and may have filed or may file patent applications and may have been issued or may be issued patents with claims that overlap or conflict with our patent applications, either by claiming the same compositions, formulations or methods or by claiming subject matter that could dominate our patent position. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. As a result, any patents we may obtain in the future may not provide us with adequate and continuing patent protection sufficient to exclude others from commercializing products similar to our products and product candidates.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain. No consistent policy regarding the breadth of claims allowed in biotechnology and pharmaceutical patents has emerged to date in the United States or in many foreign jurisdictions. In addition, the determination of patent rights with respect to pharmaceutical compounds commonly involves complex legal and factual questions, which has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our competitors may also seek approval to market their own products similar to or otherwise competitive with our products. Alternatively, our competitors may seek to market generic versions of any approved products by submitting ANDAs to the FDA in which they claim that our patents are invalid, unenforceable or not infringed. In these circumstances, we may need to defend or assert our patents, or both, including by filing lawsuits alleging patent infringement. In any of these types of proceedings, a court or other agency with jurisdiction may find our patents invalid or unenforceable, or that our competitors are competing in a non-infringing manner. Thus, even if we have valid and enforceable patents, these patents still may not provide protection against competing products or processes sufficient to achieve our business objectives.
In the future, one or more of our products and product candidates may be in-licensed from third parties. Accordingly, in some cases, the availability and scope of potential patent protection is limited based on prior decisions by our licensors or the inventors, such as decisions on when to file patent applications or whether to file patent applications at all. Our failure to obtain, maintain, enforce or defend such intellectual property rights, for any reason, could allow third parties, in particular, other established and better financed competitors having established development, manufacturing and distribution capabilities, to make competing products or impact our ability to develop, manufacture and market our products and product candidates, even if approved, on a commercially viable basis, if at all, which could have a material adverse effect on our business.
In addition to patent protection, we expect to rely heavily on trade secrets, know-how and other unpatented technology, which are difficult to protect. Although we seek such protection in part by entering into confidentiality agreements with our vendors, employees, consultants and others who may have access to proprietary information, we cannot be certain that these agreements will not be breached, adequate remedies for any breach would be available, or our trade secrets, know-how and other unpatented proprietary technology will not otherwise become known to or be independently developed by our competitors. If we are unsuccessful in protecting our intellectual property rights, sales of our products may suffer and our ability to generate revenue could be severely impacted.
| -64- |
Issued patents covering our products and product candidates could be found invalid or unenforceable if challenged in court or in administrative proceedings. We may not be able to protect our trade secrets in court.
If we initiate legal proceedings against a third-party to enforce a patent covering one of our products or product candidates, should such a patent issue, the defendant could counterclaim that the patent covering our product or product candidate is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, written description or non- enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld information material to patentability from the USPTO, or made a misleading statement, during prosecution. Third parties also may raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re- examination, post grant review, inter partes review and equivalent proceedings in foreign jurisdictions. An adverse determination in any of the foregoing proceedings could result in the revocation or cancellation of, or amendment to, our patents in such a way that they no longer cover our products or product candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which the patent examiner and we were unaware during prosecution. If a defendant or third party were to prevail on a legal assertion of invalidity or unenforceability, we could lose at least part, and perhaps all, of the patent protection on one or more of our products and product candidates. Such a loss of patent protection could have a material adverse impact on our business.
In addition, our trade secrets may otherwise become known or be independently discovered by competitors. Competitors and other third parties could purchase our products and product candidates and attempt to replicate some or all of the competitive advantages we derive from our development efforts, willfully infringe, misappropriate or otherwise violate our intellectual property rights, design around our protected technology or develop their own competitive technologies that fall outside of our intellectual property rights. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor or other third party, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If our trade secrets are not adequately protected or sufficient to provide an advantage over our competitors, our competitive position could be adversely affected, as could our business. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating our trade secrets.
We may be subject to claims challenging the inventorship or ownership of the patents and other intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an ownership interest in the patents and intellectual property that we own or that we may own or license in the future. While it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own or such assignments may not be self-executing or may be breached. We could be subject to ownership disputes arising, for example, from conflicting obligations of employees, consultants or others who are involved in developing our products or product candidates. Litigation may be necessary to defend against any claims challenging inventorship or ownership. If we or fail in defending any such claims, we may have to pay monetary damages and may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property, which could adversely impact our business, results of operations and financial condition.
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non- compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and applications are required to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and applications. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process and after a patent has issued. There are situations in which non- compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. The terms of one or more licenses that we enter into the future may not provide us with the ability to maintain or prosecute patents in the portfolio, and must therefore rely on third parties to do so.
| -65- |
If we do not obtain patent term extension and data exclusivity for our products and product candidates, our business may be materially harmed.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired for a product candidate, we may be open to competition from competitive products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
In the future, if we obtain an issued patent covering one of our present or future product candidates, depending upon the timing, duration and specifics of any FDA marketing approval of such product candidates, such patent may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, or Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent extension term of up to five years as compensation for patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent may be extended and only those claims covering the approved drug, a method for using it or a method for manufacturing it may be extended. A patent may only be extended once and only based on a single approved product. However, we may not be granted an extension because of, for example, failure to obtain a granted patent before approval of a product candidate, failure to exercise due diligence during the testing phase or regulatory review process, failure to apply within applicable deadlines, failure to apply prior to expiration of relevant patents or otherwise our failure to satisfy applicable requirements. A patent licensed to us by a third party may not be available for patent term extension. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration, and our revenue could be reduced, possibly materially.
Changes in patent law in the United States and other jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our products and product candidates.
Changes in either the patent laws or the interpretation of the patent laws in the United States or other jurisdictions could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. When implemented, the Leahy-Smith Act included several significant changes to U.S. patent law that impacted how patent rights could be prosecuted, enforced and defended. In particular, the Leahy-Smith Act also included provisions that switched the United States from a “first-to-invent” system to a “first-to-file” system, allowed third- party submission of prior art to the USPTO during patent prosecution and set forth additional procedures to attack the validity of a patent by the USPTO administered post grant proceedings. Under a first-to-file system, assuming the other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to the patent on an invention regardless of whether another inventor had made the invention earlier. The USPTO developed new regulations and procedures governing the administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. It remains unclear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business.
In addition, the patent positions of companies in the development and commercialization of biologics and pharmaceuticals are particularly uncertain. Recent rulings from the U.S. Court of Appeals for the Federal Circuit and the U.S. Supreme Court have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our existing patent portfolio and our ability to protect and enforce our intellectual property in the future.
| -66- |
We cannot assure you that our efforts to seek patent protection for one or more of our products and product candidates will not be negatively impacted by the decisions described above, rulings in other cases or changes in guidance or procedures issued by the USPTO. We cannot fully predict what impact courts’ decisions in historical and future cases may have on the ability of life science companies to obtain or enforce patents relating to their products in the future. These decisions, the guidance issued by the USPTO and rulings in other cases or changes in USPTO guidance or procedures could have a material adverse effect on our existing patent rights and our ability to protect and enforce our intellectual property in the future.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, maintaining, defending and enforcing patents on products and product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States could be less extensive than those in the United States. The requirements for patentability may differ in certain countries, particularly in developing countries; thus, even in countries where we do pursue patent protection, there can be no assurance that any patents will issue with claims that cover our products. There can be no assurance that we will obtain or maintain patent rights in or outside the United States under any future license agreements. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, even in jurisdictions where we pursue patent protection, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not pursued and obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and product candidates and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology and pharmaceutical products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. Proceedings to enforce our patent rights, even if obtained, in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. While we intend to protect our intellectual property rights in major markets for our products, we cannot ensure that we will be able to initiate or maintain similar efforts in all jurisdictions in which we may wish to market our products. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop.
If we are sued for infringing intellectual property rights of third parties, such litigation could be costly and time consuming and could prevent or delay us from developing or commercializing our product candidates.
Our commercial success depends, in part, on our ability to develop, manufacture, market and sell our product candidates without infringing the intellectual property and other proprietary rights of third parties. Third parties may have U.S. and non-U.S. issued patents and pending patent applications relating to compounds, methods of manufacturing compounds and/or methods of use for the treatment of the disease indications for which we are developing our product candidates. If any third-party patents or patent applications are found to cover our product candidates or their methods of use or manufacture, we and our collaborators or sublicensees may not be free to manufacture or market our product candidates as planned without obtaining a license, which may not be available on commercially reasonable terms, or at all. We may also be required to indemnify our collaborators or sublicensees in such an event.
| -67- |
There is a substantial amount of intellectual property litigation in the biotechnology and pharmaceutical industries, and we may become party to, or threatened with, litigation or other adversarial proceedings regarding intellectual property rights with respect to our products candidates, including interference and post-grant proceedings before the USPTO. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the composition, use or manufacture of our product candidates. We cannot guarantee that any of our patent searches or analyses including, but not limited to, the identification of relevant patents, the scope of patent claims or the expiration of relevant patents are complete or thorough, nor can we be certain that we have identified each and every patent and pending application in the United States and abroad that is relevant to or necessary for the commercialization of our product candidates in any jurisdiction. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our product candidates may be accused of infringing. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. Accordingly, third parties may assert infringement claims against us based intellectual property rights that exist now or arise in the future. The outcome of intellectual property litigation is subject to uncertainties that cannot be adequately quantified in advance. The pharmaceutical and biotechnology industries have produced a significant number of patents, and it may not always be clear to industry participants, including us, which patents cover various types of products or methods of use or manufacture. The scope of protection afforded by a patent is subject to interpretation by the courts, and the interpretation is not always uniform. If we were sued for patent infringement, we would need to demonstrate that our product candidates, products or methods either do not infringe the patent claims of the relevant patent or that the patent claims are invalid or unenforceable, and we may not be able to do this. Proving invalidity is difficult. For example, in the United States, proving invalidity requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel could be diverted in pursuing these proceedings, which could significantly harm our business and operating results. In addition, we may not have sufficient resources to bring these actions to a successful conclusion.
If we are found to infringe a third party’s intellectual property rights, we could be forced, including by court order, to cease developing, manufacturing or commercializing the infringing product candidate or product. Alternatively, we may be required to obtain a license from such third party in order to use the infringing technology and continue developing, manufacturing or marketing the infringing product candidate or product.
However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us; alternatively or additionally it could include terms that impede or destroy our ability to compete successfully in the commercial marketplace. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Many of our current and former employees, including our senior management, were previously employed at universities or at other biotechnology or pharmaceutical companies, including some which may be competitors or potential competitors. Some of these employees may be subject to proprietary rights, non-disclosure and non- competition agreements, or similar agreements, in connection with such previous employment. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such third party. Litigation may be necessary to defend against such claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel or sustain damages. Such intellectual property rights could be awarded to a third party, and we could be required to obtain a license from such third party to commercialize our technology or products. Such a license may not be available on commercially reasonable terms or at all. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
| -68- |
In addition, while we typically require our employees, consultants and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own, which may result in claims by or against us related to the ownership of such intellectual property. If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our senior management and scientific personnel.
We may become involved in lawsuits to protect or enforce our patents and other intellectual property rights, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents, trademarks, copyrights or other intellectual property. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming and divert the time and attention of our management and scientific personnel. In addition, our patents may become, involved in inventorship, priority, or validity disputes. To counter or defend against such claims can be expensive and time-consuming, and our adversaries may have the ability to dedicate substantially greater resources to prosecuting these legal actions than we can. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents, in addition to counterclaims asserting that our patents are invalid or unenforceable, or both.
In an infringement proceeding, a court may decide that a patent is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating intellectual property rights we own or control. An adverse result in any litigation proceeding could put one or more of our owned or in-licensed patents at risk of being invalidated or interpreted narrowly. Further, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
Even if resolved in our favor, the court may decide not to grant an injunction against further infringing activity and instead award only monetary damages, which may or may not be an adequate remedy. Litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and could distract our personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution activities.
We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
If we fail to comply with our obligations under any future intellectual property licenses with third parties, we could lose license rights that are important to our business.
In connection with our efforts to build our product candidate pipeline, we may enter into license agreements in the future. We expect that such license agreements will impose, various diligence, milestone payment, royalty, insurance and other obligations on us. If we fail to comply with our obligations under these licenses, our licensors may have the right to terminate these license agreements, in which event we might not be able to market any product that is covered by these agreements, or our licensors may convert the license to a non-exclusive license, which could negatively impact the value of the product candidate being developed under the license agreement. Termination of these license agreements or reduction or elimination of our licensed rights may also result in our having to negotiate new or reinstated licenses with less favorable terms.
| -69- |
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our marks of interest and our business may be adversely affected.
Our trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We rely on both registration and common law protection for our trademarks. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names, which we need for name recognition by potential partners or customers in our markets of interest. During trademark registration proceedings, we may receive rejections. Although we would be given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively and our business may be adversely affected.
Risks Related to Employee Matters and Managing Growth
We only have a limited number of employees to manage and operate our business.
As of September 30, 2022, we had about 12 full-time employees. Additionally, we utilize independent contractors and other third parties to assist with various aspects of our business. Our focus on the development of our product candidates requires us to optimize cash utilization and to manage and operate our business in a highly efficient manner. We cannot assure you that we will be able to hire or retain adequate staffing levels to develop our product candidates or run our operations or to accomplish all of the objectives that we otherwise would seek to accomplish.
Cyber-attacks or other failures in telecommunications or information technology systems could result in information theft, data corruption and significant disruption of our business operations.
We utilize information technology, or IT, systems and networks to process, transmit and store electronic information in connection with our business activities. As use of digital technologies has increased, cyber incidents, including deliberate attacks and attempts to gain unauthorized access to computer systems and networks, have increased in frequency and sophistication. These threats pose a risk to the security of our systems and networks, the confidentiality and the availability and integrity of our data.
Our future success depends on our ability to retain key employees, consultants and advisors and to attract, retain and motivate qualified personnel.
We are highly dependent on principal members of our executive team and key employees, the loss of whose services may adversely impact the achievement of our objectives. While we have entered into employment agreements with certain of our executive officers, any of them could leave our employment at any time. We do not maintain “key person” insurance policies on the lives of these individuals or the lives of any of our other employees. The loss of the services of one or more of our current employees might impede the achievement of our research, development and commercialization objectives. Furthermore, replacing executive officers or other key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to develop, gain marketing approval of and commercialize products successfully.
Recruiting and retaining other qualified employees, consultants and advisors for our business, including scientific and technical personnel, will also be critical to our success. There is currently a shortage of skilled executives in our industry, which is likely to continue. As a result, competition for skilled personnel is intense and the turnover rate can be high. We may not be able to attract and retain personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for individuals with similar skill sets. In addition, failure to succeed in preclinical or clinical trials may make it more challenging to recruit and retain qualified personnel.
| -70- |
In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by other entities and may have commitments under consulting or advisory contracts with those entities that may limit their availability to us. If we are unable to continue to attract and retain highly qualified personnel, our ability to develop and commercialize our product candidates will be limited.
The inability to recruit or the loss of the services of any executive, key employee, consultant or advisor may impede the progress of our research, development and commercialization objectives.
Our employees, independent contractors, consultants, collaborators and contract research organizations may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements, which could cause significant liability for us and harm our reputation.
We are exposed to the risk that our employees, independent contractors, consultants, collaborators and contract research organizations may engage in fraudulent conduct or other illegal activity. Misconduct by those parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that violates: (1) FDA regulations or similar regulations of comparable non-U.S. regulatory authorities, including those laws requiring the reporting of true, complete and accurate information to such authorities, (2) manufacturing standards, (3) federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable non-U.S. regulatory authorities, and (4) laws that require the reporting of financial information or data accurately. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self- dealing, bribery and other abusive practices. These laws and regulations restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee or collaborator misconduct could also involve the improper use of, including trading on, information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. While we have a code of conduct and business ethics, it is not always possible to identify and deter misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws, standards or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, additional reporting requirements and/or oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, imprisonment, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could have a material adverse effect on our ability to operate our business and our results of operations.
We expect to expand our organization, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of drug manufacturing, regulatory affairs and sales, marketing and distribution, as well as to support our public company operations. To manage these growth activities, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Our management may need to devote a significant amount of its attention to managing these growth activities. Moreover, our expected growth could require us to relocate to geographic areas beyond those where we have been historically located. For example, we maintain an office in Princeton, New Jersey, at which many of our finance, management and administrative personnel work. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion or relocation of our operations, retain key employees, or identify, recruit and train additional qualified personnel. Our inability to manage the expansion or relocation of our operations effectively may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could also require significant capital expenditures and may divert financial resources from other projects, such as the development of additional product candidates. If we are unable to effectively manage our expected growth, our expenses may increase more than expected, our ability to generate revenues could be reduced and we may not be able to implement our business strategy, including the successful commercialization of our product candidates.
| -71- |
Risks Related to Our Common Stock
The market price of our common stock may be significantly volatile.
The market price for our common stock may be volatile and subject to wide fluctuations in response to factors including the following:
| ● | actual or anticipated fluctuations in our quarterly or annual operating results; | |
| ● | changes in financial or operational estimates or projections; | |
| ● | conditions in markets generally; | |
| ● | changes in the economic performance or market valuations of companies similar to ours; and | |
| ● | general economic or political conditions in the United States or elsewhere. |
In particular, the market prices of biotechnology companies like ours have been highly volatile due to factors, including, but not limited to:
| ● | any delay or failure to conduct a clinical trial for our product or receive approval from the FDA and other regulatory agencies; | |
| ● | developments or disputes concerning a company’s intellectual property rights; | |
| ● | technological innovations of such companies or their competitors; | |
| ● | changes in market valuations of similar companies; | |
| ● | announcements by such companies or their competitors of significant contracts, acquisitions, strategic partnerships, joint ventures, capital commitments, new technologies, or patents; and | |
| ● | failure to complete significant transactions or collaborate with vendors in manufacturing a product. |
The securities market has from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of shares of our common stock.
We do not expect to pay cash dividends in the foreseeable future and therefore investors should not anticipate cash dividends on their investment.
Our board of directors does not intend to pay cash dividends in the foreseeable future but instead intends to retain any and all earnings to finance the growth of the business. To date, we have not paid any cash dividends and there can be no assurance that cash dividends will ever be paid on our common stock.
We incur significant costs and devote substantial management time as a result of operating as a public company, and we expect those costs to increase.
As a public company, we incur significant legal, accounting and other expenses. For example, we are required to comply with certain of the requirements of the Sarbanes-Oxley Act and the Dodd-Frank Wall Street Reform and Consumer Protection Act, as well as rules and regulations subsequently implemented by the SEC, including the establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. We expect that compliance with these requirements will increase our legal and financial compliance costs and will make some activities more time consuming and costly. In addition, we expect that our management and other personnel will need to divert attention from operational and other business matters to devote substantial time to these public company requirements. In particular, we expect to incur significant expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404 of the Sarbanes-Oxley Act. We currently do not have an internal audit function, and we may need to hire or contract for additional accounting and financial staff with appropriate public company experience and technical accounting knowledge.
| -72- |
There may be limitations on the effectiveness of our internal controls, and a failure of our control systems to prevent error or fraud may materially harm our company.
We are required, pursuant to Section 404 of the Sarbanes-Oxley Act, to furnish a report by our management on, among other things, the effectiveness of our internal control over financial reporting. This assessment will need to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
Effective internal control over financial reporting is necessary for us to provide reliable and timely financial reports and, together with adequate disclosure controls and procedures, are designed to reasonably detect and prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. Undetected material weaknesses in our internal control over financial reporting could lead to financial statement restatements and require us to incur the expense of remediation.
Moreover, we do not expect that disclosure controls or internal control over financial reporting will prevent all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. Failure of our control systems to detect or prevent error or fraud could materially adversely impact us.
Any of the foregoing occurrences, should they come to pass, could negatively impact the public perception of our company, which could have a negative impact on our stock price.
We may be unable to complete our analysis of our internal controls over financial reporting in a timely manner, or these internal controls may not be determined to be effective, which may adversely affect investor confidence in our company and, as a result, the value of our common stock.
We may not be able to complete our evaluation and testing of our internal control over financial reporting. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal controls are effective.
If we are unable to assert that our internal control over financial reporting is effective, or, if applicable, our independent registered public accounting firm is unable to express an opinion on the effectiveness of our internal controls, we could lose investor confidence in the accuracy and completeness of our financial reports, which would cause the price of our common stock to decline, and we may be subject to investigation or sanctions by the SEC. We will also be required to disclose changes made in our internal control and procedures on a quarterly basis.
Anti-takeover provisions under Delaware law could make an acquisition of the combined company more difficult and may prevent attempts by the combined company stockholders to replace or remove the combined company management.
Because the combined company will be incorporated in Delaware, it is governed by the provisions of Section 203 of the Delaware General Corporation Law, or the DGCL, which prohibits stockholders owning in excess of 15% of the outstanding combined company voting stock from merging or combining with the combined company. Although we believe these provisions collectively will provide for an opportunity to receive higher bids by requiring potential acquirers to negotiate with the combined company’s board of directors, they would apply even if the offer may be considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by the combined company’s stockholders to replace or remove then current management by making it more difficult for stockholders to replace members of the board of directors, which is responsible for appointing the members of management.
| -73- |
Director and officer liability is limited.
As permitted by Delaware law, our bylaws limit the liability of our directors for monetary damages for breach of a director’s fiduciary duty except for liability in certain instances. As a result of our bylaw provisions and Delaware law, stockholders may have limited rights to recover against directors for breach of fiduciary duty.
General Risk Factors
Cyber-attacks or other failures in telecommunications or information technology systems could result in information theft, data corruption and significant disruption of our business operations.
We utilize information technology, or IT, systems and networks to process, transmit and store electronic information in connection with our business activities. As use of digital technologies has increased, cyber incidents, including deliberate attacks and attempts to gain unauthorized access to computer systems and networks, have increased in frequency and sophistication. These threats pose a risk to the security of our systems and networks, the confidentiality and the availability and integrity of our data.
Our common stock could be further diluted as the result of the issuance of additional shares of common stock, convertible securities, warrants or options.
In the past, we have issued common stock, convertible securities (such as convertible notes) and warrants in order to raise capital. We have also issued common stock as compensation for services and incentive compensation for our employees, directors and certain vendors. We have shares of common stock reserved for issuance upon the exercise of certain of these securities and may increase the shares reserved for these purposes in the future. Our issuance of additional common stock, convertible securities, options and warrants could affect the rights of our stockholders, could reduce the market price of our common stock or could result in adjustments to exercise prices of outstanding warrants (resulting in these securities becoming exercisable for, as the case may be, a greater number of shares of our common stock), or could obligate us to issue additional shares of common stock to certain of our stockholders.
Shares eligible for future sale may adversely affect the market.
From time to time, certain of our stockholders may be eligible to sell all or some of their shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144 promulgated under the Securities Act, subject to certain limitations. In general, pursuant to Rule 144, stockholders who have been non-affiliates for the preceding three months may sell shares of our common stock freely after six months subject only to the current public information requirement. Affiliates may sell shares of our common stock after six months subject to the Rule 144 volume, manner of sale, current public information and notice requirements. Any substantial sales of our common stock pursuant to Rule 144 may have a material adverse effect on the market price of our common stock.
Item 1B. Unresolved Staff Comments.
Not applicable.
Item 2. Properties.
The Company relies on short term office use contracts to procure office and meeting space.
Item 3. Legal Proceedings.
We are not currently subject to any material legal proceedings. However, we may from time to time become a party to various legal proceedings arising in the ordinary course of our business.
Item 4. Mine Safety Disclosures.
Not applicable.
| -74- |
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
The Company’s common stock trades on The Nasdaq Capital Market under the symbol “SONN.”
Holders
As of December 14, 2022, we had 132 holders of record of our common stock. The number of record holders was determined from the records of the transfer agent and does not include beneficial owners of common stock whose shares are held in the names of various security brokers, dealers, and registered clearing agencies. The transfer agent of our common stock is Securities Transfer Corporation, 2901 N Dallas Parkway, Suite 380, Plano, TX 75093.
Dividends
We have never declared or paid cash dividends on our common stock. We do not intend to declare or pay cash dividends on our common stock for the foreseeable future, but currently intend to retain any future earnings to fund the development and growth of our business. The payment of cash dividends if any, on the common stock will rest solely within the discretion of our board of directors and will depend, among other things, upon our earnings, capital requirements, financial condition, and other relevant factors.
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) is intended to help facilitate an understanding of our financial condition and its historical results of operations for the periods presented. This MD&A should be read in conjunction with the audited consolidated financial statements and notes thereto included in this annual report on Form 10-K. This MD&A may contain forward-looking statements that involve risks and uncertainties. For a discussion on forward-looking statements, see the information set forth above under the caption “Special Note Regarding Forward-Looking Statements”, which information is incorporated herein by reference.
Overview
Sonnet BioTherapeutics Holdings, Inc. (“Sonnet,” “we,” “us,” “our” or the “Company”), is a clinical stage, oncology-focused biotechnology company with a proprietary platform for innovating biologic medicines of single- or bi-specific action. Known as FHAB™ (Fully Human Albumin Binding), the technology utilizes a fully human single chain antibody fragment that binds to and “hitch-hikes” on human serum albumin for transport to target tissues. We designed the construct to improve drug accumulation in specific tissues, as well as to extend the duration of activity in the body. FHAB development candidates are produced in a mammalian cell culture, which enables glycosylation, thereby reducing the risk of immunogenicity. We believe our FHAB technology, for which we received a U.S. patent in June 2021, is a distinguishing feature of our biopharmaceutical platform that is well suited for future drug development across a range of human disease areas, including in oncology, autoimmune, pathogenic, inflammatory, and hematological conditions.
Our current internal pipeline development activities are focused on cytokines, a class of cell signaling peptides that, among other important functions, serve as potent immunomodulatory agents. Working both independently and synergistically, specific cytokines have shown the ability to modulate the activation and maturation of immune cells that fight cancer and pathogens. However, because they do not preferentially accumulate in specific tissues and are quickly eliminated from the body, the conventional approach to achieving a treatment effect with cytokine therapy typically requires the administration of high and frequent doses. This can result in a reduced treatment effect accompanied by the potential for systemic toxicity, which poses challenges to the therapeutic application of this class of drugs.
| -75- |
Our lead proprietary asset, SON-1010, is a fully human version of Interleukin 12 (“IL-12”), covalently linked to the FHAB construct, for which we intend to pursue clinical development in solid tumor indications, including non-small cell lung cancer and head and neck cancer. We have completed a nonhuman primate (“NHP”) GLP toxicity study and have successfully manufactured drug product for clinical use. In March 2022, the FDA cleared our Investigational New Drug (“IND”) application for SON-1010. This allowed us to initiate a U.S. clinical trial (SB101) in oncology patients with solid tumors during the second calendar quarter of 2022. In September 2021, we created a wholly-owned Australian subsidiary, SonnetBio Pty Ltd, for the purpose of conducting certain clinical trials. We received approval and initiated an Australian clinical study (SB102) of SON-1010 in healthy volunteers during the third calendar quarter of 2022. Initial safety and tolerability data from the SB101 and SB102 studies was presented during the calendar fourth quarter of 2022, and initial pharmacokinetic and pharmacodynamic data from both studies is expected during the second calendar quarter of 2023.
We acquired the global development rights to our most advanced compound, SON-080, a fully human version of Interleukin 6 (“IL-6”), in April 2020 through our acquisition of the outstanding shares of Relief Therapeutics SA. We are advancing SON-080 in target indications of Chemotherapy-Induced Peripheral Neuropathy (“CIPN”) and Diabetic Peripheral Neuropathy (“DPN”). We received approval to initiate an ex-U.S. Phase 1b/2a study with SON-080 in CIPN. This study could yield initial clinical safety data during the first half of 2023. Pursuant to a license agreement we entered with New Life Therapeutics Pte, Ltd. (“New Life”) of Singapore in May 2021, we and New Life will be jointly responsible for developing SON-080 in DPN. The objective will be to initiate a Phase 2 study in the second half of 2023, once the CIPN safety data is available.
SON-1210 (“IL12-FHAB-IL15”), our lead bi-specific construct, combines FHAB with fully human IL-12 and fully human Interleukin 15 (“IL-15”). This compound is being developed for solid tumor indications, including colorectal cancer. An NHP study is on track to complete in the fourth calendar quarter of 2022, and we expect to initiate the regulatory authorization process in the first half of 2023. Manufacturing and drug supply are secured with a lyophilized drug product formulation being prepared during the first calendar quarter of 2023, which is expected to be suitable for first-in-human clinical trials.
Cell line development and process development of SON-1410 (IL18-FHAB-IL12), a bi-specific combination of Interleukin 18 (“IL-18”) and IL-12, is ongoing, with early experimental drug supply suitable for formulation and analytical method development activities, in addition to small quantities for use in early development proof-of-concept in vitro studies. Process development activities will continue through 2023, with the potential to generate drug suitable for initial in vivo mouse studies by the end of the 2023 calendar year.
We have completed sequence confirmation for SON-3015 (anti-IL6-FHAB-anti-TGFβ). Early-stage bi-specific drug has been generated and has been stored for future use in in vivo mice studies.
We have incurred recurring operating losses and negative cash flows from operations since inception. Our ability to generate product or licensing revenue sufficient to achieve profitability will depend heavily on the successful development and eventual commercialization of one or more of our current or future product candidates. Our net losses were $29.7 million and $25.0 million for the years ended September 30, 2022 and 2021, respectively. As of September 30, 2022, we had cash of $3.1 million. We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. We expect that our expenses and capital requirements will increase substantially in connection with our ongoing activities, particularly if and as we:
| ● | conduct additional clinical trials for product candidates; | |
| ● | continue to discover and develop additional product candidates; | |
| ● | acquire or in-license other product candidates and technologies; | |
| ● | maintain, expand and protect our intellectual property portfolio; |
| -76- |
| ● | hire additional clinical, scientific and commercial personnel; | |
| ● | establish a commercial manufacturing source and secure supply chain capacity sufficient to provide commercial quantities of any product candidates for which we may obtain regulatory approval; | |
| ● | seek regulatory approval for product candidates that successfully complete clinical trials; | |
| ● | establish a sales, marketing and distribution infrastructure to commercialize any products for which we may obtain regulatory approval; and | |
| ● | add operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts, as well as to support our operation as a public reporting company. |
We will not generate revenue from product sales, if any, unless and until we receive licensing revenue and/or successfully complete clinical development and obtain regulatory approval for our product candidates. If we obtain regulatory approval for any of our product candidates and do not enter into a commercialization partnership, we expect to incur significant expenses related to developing our internal commercialization capability to support product sales, marketing and distribution. We will continue to incur significant costs associated with operating as a public company.
As a result, we will need substantial additional funding to support our continuing operations and pursue our growth strategy. Until such time as we can generate significant revenue from product sales, if ever, we expect to finance our operations through the sale of equity, debt financings or other capital sources, which may include collaborations with other companies or other strategic transactions. We may not be able to raise additional funds or enter into such other agreements or arrangements when needed on favorable terms, or at all. If we fail to raise capital or enter into such agreements as and when needed, we may have to significantly delay, reduce or eliminate the development and commercialization of one or more of our product candidates or delay our pursuit of potential in-licenses or acquisitions.
Because of the numerous risks and uncertainties associated with product development, we are unable to predict the timing or amount of increased expenses or when or if we will be able to achieve or maintain profitability. Even if we are able to generate product sales, we may not become profitable. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce or terminate operations.
Since our inception in 2015, we have devoted substantially all of our efforts and financial resources to organizing and staffing the Company, business planning, raising capital, acquiring or discovering product candidates and securing related intellectual property rights and conducting discovery, research and development activities for product candidates. We do not have any products approved for sale and have not generated any revenue from product sales. We have funded our operations to date primarily with proceeds from sales of common stock, warrants and proceeds from the issuance of convertible debt.
COVID-19 Pandemic
In December 2019, a novel strain of coronavirus, COVID-19, was reported to have surfaced in Wuhan, China and on March 11, 2020 was declared a pandemic by the World Health Organization. Many countries around the world imposed quarantines and restrictions on travel and mass gatherings to slow the spread of COVID-19 and closed non-essential businesses. As countries and state and local jurisdictions continue to put restrictions in place, our ability to continue to operate our business may also be limited. Such events may result in a period of business, supply and drug product manufacturing disruption, and in reduced operations, any of which could materially affect our business, financial condition and results of operations.
This pandemic or outbreak could result in difficulty securing clinical trial site locations, CROs, and/or trial monitors and other critical vendors and consultants supporting the trial. In addition, outbreaks or the perception of an outbreak near a clinical trial site location could impact our ability to enroll patients. These situations, or others associated with COVID-19, could cause delays in our clinical trial plans and could increase expected costs, all of which could have a material adverse effect on our business and its financial condition.
| -77- |
In particular, manufacturing of our pipeline products (other than SON-1010) has been delayed by COVID-19 related supply chain issues, specifically supply of raw materials, including media, resins, and analytical kits, compounded by international shipping delays.
While the potential economic impact brought by, and the duration of, COVID-19 may be difficult to assess or predict, a widespread pandemic could result in significant disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect our liquidity. In addition, a recession or market correction resulting from the spread of COVID-19 could materially affect our business and the value of our common shares.
The COVID-19 outbreak may also affect the ability of our staff and the parties we work with to carry out our non-clinical, clinical, and drug manufacturing activities. We rely or may in the future rely on clinical sites, investigators and other study staff, consultants, independent contractors, contract research organizations and other third-party service providers to assist us in managing, monitoring and otherwise carrying out our non-clinical studies and clinical trials. We also rely or may in the future rely on consultants, independent contractors, contract manufacturing organizations, and other third-party service providers to assist us in managing, monitoring and otherwise carrying out our API production, formulation, and drug manufacturing activities. COVID-19 may affect the ability of any of these external people, organizations, or companies to devote sufficient time and resources to our programs or to travel to perform work for us.
Potential negative impacts of the COVID-19 outbreak on the conduct of current or future clinical studies include delays in gaining feedback from regulatory agencies, starting new clinical studies, and recruiting subjects to studies that are enrolling. The potential negative impacts also include inability to have study visits at study sites, incomplete collection of safety and efficacy data, and higher rates of drop-out of subjects from ongoing studies, delays in site entry of study data into the data base, delays in monitoring of study data because of restricted physical access to study sites, delays in site responses to queries, delays in data-base lock, delays in data analyses, delays in time to top-line data, and delays in completing study reports. New or worsening COVID-19 disruptions or restrictions could have the potential to further negatively impact our non-clinical studies, clinical trials, and drug manufacturing activities. At the current time, we are unable to quantify the potential effects of this pandemic on future operations.
Components of Results of Operations
Collaboration Revenue
Collaboration revenue is currently earned from the license arrangement entered into with New Life in May 2021 (“New Life Agreement”), which granted New Life rights to an exclusive license (with the right to sublicense) to develop and commercialize pharmaceutical preparations containing a specific recombinant human interleukin-6, SON-080 (the “Compound”) (such preparations, the “Products”) for the prevention, treatment or palliation of diabetic peripheral neuropathy in humans (the “DPN Field”) in the Exclusive Territory. We identified the following obligations under the arrangement: (i) License to develop, market, import, use and commercialize the Product in the Field in the Exclusive Territory (the “License”); and (ii) transfer of know-how and clinical development and regulatory activities (“R&D Activities”). We determined that the License and the R&D Activities are not distinct from each other and, therefore, combined these material promises into a single performance obligation. Under this agreement, we received upfront cash payments totaling $1.0 million, which were fully allocated to the single performance obligation and are being recognized over the estimated performance period of R&D services.
| -78- |
Operating Expenses
Research and Development Expenses
Research and development expenses consist primarily of costs incurred in connection with the discovery and development of our product candidates. We expense research and development costs as incurred and such costs include:
| ● | employee-related expenses, including salaries, share-based compensation and related benefits, for employees engaged in research and development functions; | |
| ● | expenses incurred in connection with the preclinical and clinical development of our product candidates, including under agreements with third parties, such as consultants and clinical research organizations; | |
| ● | the cost of manufacturing drug products for use in our preclinical studies and clinical trials, including under agreements with third parties, such as consultants and contract manufacturing organizations; | |
| ● | facilities, depreciation and other expenses, which include direct or allocated expenses for rent and maintenance of facilities and insurance; | |
| ● | costs related to compliance with regulatory requirements; and | |
| ● | payments made under third-party licensing agreements. |
We recognize external development costs based on an evaluation of the progress to completion of specific tasks using information provided by our service providers. This process involves reviewing open contracts and purchase orders, communicating with their personnel to identify services that have been performed on our behalf, and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual costs. Nonrefundable advance payments for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses. Such amounts are recognized as an expense when the goods have been delivered or the services have been performed.
Our direct research and development expenses consist primarily of external costs, such as fees paid to outside consultants, CROs, CMOs and research laboratories in connection with preclinical development, process development, manufacturing and clinical development activities. Our direct research and development expenses also include fees incurred under third-party license agreements. We do not allocate employee costs and costs associated with discovery efforts, laboratory supplies and facilities, including depreciation or other indirect costs, to specific product candidates because these costs are deployed across multiple programs and as such, are not separately classified. We use internal resources primarily to conduct research and discovery as well as for managing preclinical development, process development, manufacturing and clinical development activities. These employees work across multiple programs and therefore, we do not track costs by product candidate.
We expect our research and development expense will increase for the foreseeable future as we attempt to advance development of our product candidates. The successful development of our product candidates is highly uncertain. At this time, we cannot reasonably estimate or know the nature, timing and costs of the efforts that will be necessary to complete the remainder of the development of our current pipeline or any future product candidates we may develop due to the numerous risks and uncertainties associated with clinical development, including risk and uncertainties related to:
| ● | the timing and progress of preclinical and clinical development activities; | |
| ● | the number and scope of preclinical and clinical programs that we decide to pursue; | |
| ● | our ability to maintain our current research and development programs and to establish new ones; | |
| ● | establishing an appropriate safety profile with investigational new drug-enabling studies; | |
| ● | successful patient enrollment in, and the initiation and completion of, clinical trials; | |
| ● | the successful completion of clinical trials with safety, tolerability and efficacy profiles that are satisfactory to the FDA or any comparable foreign regulatory authority; | |
| ● | the receipt of regulatory approvals from applicable regulatory authorities; |
| -79- |
| ● | the timing, receipt and terms of any marketing approvals from applicable regulatory authorities; | |
| ● | our ability to establish new licensing or collaboration arrangements; | |
| ● | establishing agreements with third-party manufacturers for clinical supply for our clinical trials and commercial manufacturing, if any of our product candidates is approved; | |
| ● | development and timely delivery of clinical-grade and commercial-grade drug formulations that can be used in our clinical trials and for commercial launch; | |
| ● | obtaining, maintaining, defending and enforcing patent claims and other intellectual property rights; | |
| ● | launching commercial sales of product candidates, if approved, whether alone or in collaboration with others; | |
| ● | maintaining a continued acceptable safety profile of the product candidates following approval; and | |
| ● | the potential impact of COVID-19 on operations which may affect, among other things, the timing of clinical trials, availability of raw materials, and the ability to access and secure testing facilities. |
A change in the outcome of any of these variables with respect to the development of our product candidates could significantly change the costs and timing associated with the development of that product candidate. We may never succeed in obtaining regulatory approval for any of our product candidates.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs for personnel, including share-based compensation, in executive, finance and administrative functions. General and administrative expenses also include direct and allocated facility-related costs as well as professional fees for legal, patent, consulting, accounting, and audit services.
Our general and administrative expenses will increase in the future as we increase our headcount to support continued research activities and development of product candidates. We will continue to incur increased accounting, audit, legal, regulatory, compliance and director and officer insurance costs as well as investor and public relations expenses associated with being a public company.
Foreign Exchange Loss
Foreign exchange loss consists of exchange rate changes on transactions denominated in currencies other than the U.S. dollar.
Other Income
During 2021, we recognized other income in connection with forgiveness of our PPP loan.
| -80- |
Results of Operations
Comparison of the years ended September 30, 2022 and 2021
The following table summarizes our results of operations for the years ended September 30, 2022 and 2021:
| Years Ended September 30, | ||||||||||||
| 2022 | 2021 | Change | ||||||||||
| Collaboration revenue | $ | 349,943 | $ | 483,626 | $ | (133,683 | ) | |||||
| Operating expenses | ||||||||||||
| Research and development | 21,444,019 | 16,634,553 | 4,809,466 | |||||||||
| General and administrative | 8,575,283 | 8,936,509 | (361,226 | ) | ||||||||
| Total operating expenses | 30,019,302 | 25,571,062 | 4,448,240 | |||||||||
| Loss from operations | (29,669,359 | ) | (25,087,436 | ) | 4,314,557 | |||||||
| Interest income | — | 15 | (15 | ) | ||||||||
| Foreign exchange loss | (52,482 | ) | (22,041 | ) | (30,441 | ) | ||||||
| Other income | — | 125,501 | (125,501 | ) | ||||||||
| Net loss | $ | (29,721,841 | ) | $ | (24,983,961 | ) | $ | (4,737,880 | ) | |||
Collaboration Revenue
We recognized $0.3 million of revenue related to the New Life Agreement during the year ended September 30, 2022 compared to $0.5 million of revenue recognized during the year ended September 30, 2021.
Research and Development Expenses
Research and development expenses were $21.4 million for the year ended September 30, 2022, compared to $16.6 million for the year ended September 30, 2021. The increase of $4.8 million was primarily due to increased expenditures for the development of the cell lines for IL12-FHAB, IL12-FHAB-IL15 and SON-080 in connection with the initiation of a Phase 1 clinical trial for SON-1010 and preparation for a Phase 1b/2a pilot-scale efficacy study with SON-080 in CIPN; acquired in-process research and development the milestone payment incurred in connection with various license and research and development agreements, including the XOMA Collaboration Agreement; and an increase in payroll expense as we continue to develop our product candidates.
General and Administrative Expenses
General and administrative expenses were $8.6 million for the year ended September 30, 2022, compared to $8.9 million for the year ended September 30, 2021. The decrease of $0.4 million was primarily due to a decrease in payroll and share-based compensation expense.
Other Income
Other income of $0.1 million was recognized in connection with forgiveness of our PPP loan in June of 2021.
Liquidity and Capital Resources
We have funded operations to date primarily with proceeds from sales of common stock, warrants and proceeds from the issuance of convertible debt. We will need to sell additional securities during our fiscal year 2023. There is no certainty that equity or debt financing will be available in the future or that it will be at acceptable terms and at this time, it is not possible to predict the outcome of these matters.
We have incurred significant net losses of $29.7 million and $25.0 million for the years ended September 30, 2022 and 2021, respectively. We expect to continue to incur significant operational expenses and net losses in the upcoming 12 months and beyond. Our net losses may fluctuate significantly from quarter to quarter and year to year, depending on the stage and complexity of our R&D studies and related expenditures, the receipt of additional payments on the licensing of our technology, if any, and the receipt of payments under any current or future collaborations we may enter into.
We have evaluated whether there are conditions or events, considered in the aggregate, that raise substantial doubt about our ability to continue as a going concern. We believe our cash of $3.1 million at September 30, 2022, together with the $4.6 million of net proceeds received from the sale of common stock pursuant to the 2022 Sales Agreement (as defined below) subsequent to September 30, 2022 through December 15, 2022 will fund our projected operations into March 2023. Substantial additional financing will be needed by us to fund our operations. These factors raise substantial doubt about our ability to continue as a going concern.
| -81- |
The following table summarizes our sources and uses of cash for each of the years presented:
| Years Ended September 30, | ||||||||
| 2022 | 2021 | |||||||
| Net cash used in operating activities | $ | (27,687,278 | ) | $ | (22,552,245 | ) | ||
| Net cash used in investing activities | (896,477 | ) | (3,623 | ) | ||||
| Net cash provided by financing activities | 4,014,567 | 42,828,032 | ||||||
| Net (decrease) increase in cash | $ | (24,569,188 | ) | $ | 20,272,164 | |||
Operating Activities
During the year ended September 30, 2022, we used $27.7 million of cash in operating activities which was primarily attributable to our net loss of $29.7 million. This amount was offset by $0.9 million of share-based compensation expense and $1.0 million of acquired in-process research and development, and a $0.1 million net decrease in operating assets and liabilities.
During the year ended September 30, 2021, we used $22.6 million of cash in operating activities which was primarily attributable to our net loss of $25.0 million. This amount was offset by $1.4 million of share-based compensation expense, and a $1.0 million net decrease in operating assets and liabilities.
Investing Activities
During the year ended September 30, 2022, we used $0.9 million for the purchase of acquired in-process research and development.
During the year ended September 30 2021, we purchased $3,623 of office furniture and computer equipment.
Financing Activities
During the year ended September 30, 2022, net cash provided by financing activities was $4.0 million, consisting primarily of net proceeds from the sale of preferred stock and warrants and net proceeds from the sale of common stock.
During the year ended September 30, 2021, net cash provided by financing activities was $42.8 million, consisting primarily of net proceeds from the sale of common stock and warrants.
Funding Requirements
We expect our expenses to increase substantially in connection with our ongoing activities, particularly as we advance preclinical activities and clinical trials of product candidates in development. In addition, we expect to incur additional costs associated with operating as a public company. The timing and amount of our operating expenditures will depend largely on:
| ● | the scope, number, initiation, progress, timing, costs, design, duration, any potential delays, and results of clinical trials and nonclinical studies for our current or future product candidates; | |
| ● | the clinical development plans we establish for these product candidates; | |
| ● | the number and characteristics of product candidates and programs that we develop or may in-license; |
| -82- |
| ● | the outcome, timing and cost of regulatory reviews, approvals or other actions to meet regulatory requirements established by the FDA and comparable foreign regulatory authorities, including the potential for the FDA or comparable foreign regulatory authorities to require that we perform more studies for our product candidates than those that we currently expect; | |
| ● | our ability to obtain marketing approval for product candidates; | |
| ● | the cost of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights covering our product candidates; | |
| ● | our ability to maintain, expand and defend the scope of our intellectual property portfolio, including the cost of defending intellectual property disputes, including patent infringement actions brought by third parties against us or our product candidates; | |
| ● | the cost and timing of completion of commercial-scale outsourced manufacturing activities with respect to product candidates; | |
| ● | our ability to establish and maintain licensing, collaboration or similar arrangements on favorable terms and whether and to what extent we retain development or commercialization responsibilities under any new licensing, collaboration or similar arrangement; | |
| ● | the cost of establishing sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval in regions where we choose to commercialize our products on our own; | |
| ● | the success of any other business, product or technology that we acquire or in which we invest; | |
| ● | the costs of acquiring, licensing or investing in businesses, product candidates and technologies; | |
| ● | our need and ability to hire additional management and scientific and medical personnel; | |
| ● | the costs to operate as a public company in the United States, including the need to implement additional financial and reporting systems and other internal systems and infrastructure for our business; | |
| ● | market acceptance of our product candidates, to the extent any are approved for commercial sale; | |
| ● | the effect of competing technological and market developments; and | |
| ● | the potential impact of the COVID-19 pandemic on our clinical trials and operations. |
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances, and marketing, distribution or licensing arrangements with third parties. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of ours may be materially diluted, and the terms of such securities could include liquidation or other preferences that adversely affect the rights of our stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include restrictive covenants that limit our ability to take specified actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to technologies, future revenue streams, research programs or product candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay, reduce, cease or eliminate product development or future commercialization efforts, sell off assets, or grant rights to develop and market product candidates that we would otherwise prefer to develop and market or cease operations.
| -83- |
Private Placement of Preferred Stock
In August 2022, we entered into a securities purchase agreement (the “Preferred SPA”) with several accredited investors for the issuance and sale of (i) an aggregate of 22,275 shares of our Series 3 Convertible Preferred Stock, stated value $100 per share, (ii) 225 shares of our Series 4 Convertible Preferred Stock, stated value $100 per share, and (iii) Series 3 warrants to purchase up to 276,140 shares of our common stock in a private placement for aggregate gross proceeds of $2.3 million, with $0.1 million of issuance costs for net proceeds of $2.1 million. The shares of Series 3 Convertible Preferred Stock were convertible into an aggregate of 546,760 shares of our common stock and the shares of Series 4 Convertible Preferred Stock were convertible into an aggregate of 5,523 shares of our common stock, in each case, at a conversion price of $4.074 per share. The Series 3 warrants have an exercise price of $4.074 per share, are exercisable commencing six months after issuance, and will expire five years from the issuance date. In September 2022, all shares of preferred stock issued in connection with the Preferred SPA were converted into shares of common stock.
At-the-Market Offering of Common Stock
We entered into an At-the-Market Sales Agreement with BTIG, LLC (“BTIG”) on August 15, 2022 (the “2022 Sales Agreement”). Pursuant to the 2022 Sales Agreement, we may offer and sell, from time to time, through BTIG, as sales agent and/or principal, shares of our common stock, having an aggregate offering price of up to $25.0 million, subject to certain limitations on the amount of common stock that may be offered and sold by us set forth in the 2022 Sales Agreement. Due to the offering limitations applicable to us under General Instruction I.B.6. of Form S-3 and our public float as of August 15, 2022, and in accordance with the terms of the 2022 Sales Agreement, we could offer shares having an aggregate gross sales price of up to $6.1 million pursuant to the prospectus supplement dated August 15, 2022, filed in connection with the 2022 Sales Agreement. As of November 9, 2022, we had sold an aggregate of 2,477,287 shares of common stock for gross proceeds of $6.1 million, and we filed a prospectus supplement dated November 9, 2022 registering the offer and sale of shares of our common stock for an aggregate offering price of up to an additional $1.7 million pursuant to the 2022 Sales Agreement. As of December 14, 2022, we have sold an aggregate of 579,239 shares of common stock for gross proceeds of $0.7 million under the prospectus supplement dated November 9, 2022. We are not obligated to make any sales of shares under the 2022 Sales Agreement and any determination by us to do so will be dependent, among other things, on market conditions and our capital raising needs.
Contractual Obligations and Commitments
The following table summarizes the Company’s contractual obligations as of September 30, 2022 and the effects that such obligations are expected to have on its liquidity and cash flows in future periods:
| Less than 1 Year | 1 to 3 Years | 4 to 5 Years | More than 5 Years | Total | ||||||||||||||||
| Operating Lease (1) | $ | 79,259 | $ | 189,101 | $ | 48,216 | $ | — | $ | 316,576 | ||||||||||
| Debt Obligations (2) | 748 | — | — | — | 748 | |||||||||||||||
| Total | $ | 80,007 | $ | 189,101 | $ | 48,216 | $ | — | $ | 317,324 | ||||||||||
(1) Reflects obligations pursuant to the Company’s office lease in Princeton, New Jersey.
(2) Reflects unsecured notes payable issued to certain related parties.
In addition to the contracts with payment commitments that we have reflected in the table above, we have entered into other contracts in the normal course of business with certain CROs, CMOs and other third-parties for preclinical research studies and testing, clinical trials and manufacturing services. These contracts do not contain any minimum purchase commitments and are cancelable upon prior notice and as a result, are not included in the table of contractual obligations and commitments above. Payments due upon cancellation consist only of payments for services provided and expenses incurred, including non-cancelable obligations to our service providers, up to the date of cancellation.
| -84- |
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of financial condition and results of operations are based on our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, and expenses and the disclosure of contingent assets and liabilities in our financial statements. On an ongoing basis, we evaluate our estimates and judgments, including those related to the accrual for research and development expenses. We base our estimates on historical experience, known trends and events, and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While the Company’s significant accounting policies are described in more detail in the notes to the consolidated financial statements included elsewhere in this Form 10-K, we believe that the following accounting policies are those most critical to the judgments and estimates used in the preparation of the consolidated financial statements.
Research and development expenses
Research and development expenses include all direct and indirect costs associated with the development of the our biopharmaceutical products. These expenses include personnel costs, consulting fees, and payments to third parties for research, development, and manufacturing services. These costs are charged to expense as incurred.
At the end of each reporting period, we compare payments made to third-party service providers to the estimated progress toward completion of the related project, based on the measure of progress as defined in the contract. Factors we consider in preparing the estimates include costs incurred by the service provider, milestones achieved, and other criteria related to the efforts of our service providers. Such estimates are subject to change as additional information becomes available. Depending on the timing of payment to the third-party service providers and the progress we estimate has been made as a result of the service provided, we will record a prepaid expense or accrued liability related to these costs. Contingent development or regulatory milestone payments are recognized upon the related resolution of such contingencies. As of September 30, 2022, we did not make any material adjustments to our prior estimates of accrued research and development expenses.
Recently Issued Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position and results of operations is disclosed in Note 2 to the consolidated financial statements included elsewhere in this Form 10-K.
Item 7A. Qualitative and Quantitative Disclosures about Market Risk.
Not applicable.
| -85- |
Item 8. Financial Statements and Supplementary Data.
SONNET BIOTHERAPEUTICS HOLDINGS, INC.
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
| Page | |
| Report
of Independent Registered Public Accounting Firm (KPMG LLP, Philadelphia, PA, Auditor Firm ID: |
87 |
| Consolidated Balance Sheets | 89 |
| Consolidated Statements of Operations | 90 |
| Consolidated Statements of Changes in Stockholders’ Equity (Deficit) | 91 |
| Consolidated Statements of Cash Flows | 92 |
| Notes to Consolidated Financial Statements | 93 |
| -86- |
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
Sonnet BioTherapeutics Holdings, Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Sonnet BioTherapeutics Holdings, Inc. and subsidiaries (the Company) as of September 30, 2022 and 2021, the related consolidated statements of operations, changes in stockholders’ equity (deficit), and cash flows for the years then ended, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of September 30, 2022 and 2021, and the results of its operations and its cash flows for the years then ended, in conformity with U.S. generally accepted accounting principles.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has incurred recurring losses and negative cash flows from operations since inception and will require substantial additional financing to continue to fund its research and development activities that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
| -87- |
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Prepaid development expenses and accrued research and development expense
As discussed in Notes 2 and 3 to the consolidated financial statements, research and development costs are expensed as incurred, which include amounts due to third parties for research, development, and manufacturing services. At the end of each reporting period, the Company compares the payments made to third-party service providers to the estimated progress towards completion of the related project, based on the measure of progress as defined in the contract. Factors the Company considers in preparing the estimates include costs incurred by the service provider, milestones achieved, and other criteria related to the efforts of its service providers. Depending on the timing of payments to the third-party service providers and the progress the Company estimates has been made as a result of the services provided, the Company will record a prepaid expense or accrued liability related to these costs. As of September 30, 2022, the Company reported prepaid expenses and other current assets of $2.4 million, a portion of which related to these costs, and accrued research and development expenses of $1.6 million.
We identified the evaluation of certain prepaid and accrued research and development expenses for third-party service providers as a critical audit matter. Evaluating the estimated progress toward completion of research and development projects, including the factors described above, required especially subjective auditor judgment.
The following are the primary procedures we performed to address this critical audit matter. To evaluate the Company’s estimate of costs incurred as of September 30, 2022, for a selection of prepaid and accrued research and development expenses, we (1) examined the provisions in the contracts, invoices and communications received from third party service providers related to the project status; (2) sent confirmations to the third-party service providers; and (3) inquired of the individuals who are responsible for monitoring and tracking the status of research and development activities.
/s/
We have served as the Company’s auditor since 2015.
December 15, 2022
| -88- |
Sonnet BioTherapeutics Holdings, Inc.
Consolidated Balance Sheets
| September 30, | ||||||||
| 2022 | 2021 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash | $ | $ | ||||||
| Prepaid expenses and other current assets | ||||||||
| Total current assets | ||||||||
| Property and equipment, net | ||||||||
| Operating lease right-of-use asset | ||||||||
| Deferred offering costs | ||||||||
| Total assets | $ | $ | ||||||
| Liabilities and stockholders’ (deficit) equity | ||||||||
| Current liabilities: | ||||||||
| Related-party notes | $ | $ | ||||||
| Accounts payable | ||||||||
| Accrued expenses | ||||||||
| Operating lease liability | ||||||||
| Deferred income | ||||||||
| Total current liabilities | ||||||||
| Operating lease liability | ||||||||
| Total liabilities | ||||||||
| Commitments and contingencies (Note 5) | ||||||||
| Stockholders’ (deficit) equity: | ||||||||
| Preferred stock; $ par value: shares authorized. shares issued or outstanding | ||||||||
| Common stock; $ par value: shares authorized; and shares issued and outstanding at September 30, 2022 and 2021, respectively | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total stockholders’ (deficit) equity | ( | ) | ||||||
| Total liabilities and stockholders’ (deficit) equity | $ | $ | ||||||
See accompanying notes to consolidated financial statements
| -89- |
Sonnet BioTherapeutics Holdings, Inc.
Consolidated Statements of Operations
| Years ended September 30, | ||||||||
| 2022 | 2021 | |||||||
| Collaboration revenue | $ | $ | ||||||
| Operating expenses: | ||||||||
| Research and development | ||||||||
| General and administrative | ||||||||
| Total operating expenses | ||||||||
| Loss from operations | ( | ) | ( | ) | ||||
| Interest income | ||||||||
| Foreign exchange loss | ( | ) | ( | ) | ||||
| Other income | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Per share information: | ||||||||
| Net loss per share, basic and diluted | $ | ( | ) | $ | ( | ) | ||
| Weighted average shares outstanding, basic and diluted | ||||||||
See accompanying notes to consolidated financial statements
| -90- |
Sonnet BioTherapeutics Holdings, Inc.
Consolidated Statements of Changes in Stockholders’ Equity (Deficit)
| Preferred stock | Common stock | Additional paid-in | Accumulated | |||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | capital | deficit | Total | ||||||||||||||||||||||
| Balance at October 1, 2020 | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||||||
| Sale of common stock and warrants, net of issuance costs | — | |||||||||||||||||||||||||||
| Issuance of common stock on vesting of restricted stock units | — | ( | ) | |||||||||||||||||||||||||
| Net share settlement of warrants | — | ( | ) | |||||||||||||||||||||||||
| Warrant exercises | — | |||||||||||||||||||||||||||
| Share-based compensation | — | — | ||||||||||||||||||||||||||
| Net loss | — | — | ( | ) | ( | ) | ||||||||||||||||||||||
| Balance at September 30, 2021 | ( | ) | ||||||||||||||||||||||||||
| Sale of preferred stock and common stock warrants, net of issuance costs | — | |||||||||||||||||||||||||||
| Conversion of preferred stock into common stock | ( | ) | ( | ) | ||||||||||||||||||||||||
| Sale of common stock, net of issuance costs | — | |||||||||||||||||||||||||||
| Issuance of common stock on vesting of restricted stock units | — | ( | ) | |||||||||||||||||||||||||
| Share-based compensation | — | — | ||||||||||||||||||||||||||
| Net loss | — | — | ( | ) | ( | ) | ||||||||||||||||||||||
| Balance at September 30, 2022 | $ | $ | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||||||
See accompanying notes to consolidated financial statements
| -91- |
Sonnet BioTherapeutics Holdings, Inc.
Consolidated Statements of Cash Flows
| Years ended September 30, | ||||||||
| 2022 | 2021 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Acquired in-process research and development | ||||||||
| Depreciation | ||||||||
| Amortization of operating lease right-of-use asset | ||||||||
| Share-based compensation | ||||||||
| Non-cash interest expense | ||||||||
| Forgiveness of note payable | ( | ) | ||||||
| Change in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | ( | ) | ( | ) | ||||
| Other assets | ||||||||
| Accounts payable | ||||||||
| Accrued expenses | ||||||||
| Operating lease liability | ( | ) | ( | ) | ||||
| Deferred income | ( | ) | ||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchases of in-process research and development | ( | ) | ||||||
| Purchases of property and equipment | ( | ) | ||||||
| Net cash used in investing activities | ( | ) | ( | ) | ||||
| Cash flows from financing activities: | ||||||||
| Proceeds from sale of preferred stock and common stock warrants, net of issuance costs | ||||||||
| Proceeds from sale of common stock, net of issuance costs | ||||||||
| Proceeds from warrant exercises, net of issuance costs | ||||||||
| Repayments of related-party notes | ( | ) | ||||||
| Net cash provided by financing activities | ||||||||
| Net (decrease) increase in cash | ( | ) | ||||||
| Cash, beginning of year | ||||||||
| Cash, end of year | $ | $ | ||||||
| Supplemental disclosure of non-cash operating, investing and financing activities: | ||||||||
| Change in operating lease right-of-use asset and liability due to amended lease | $ | $ | ||||||
| Net settlement of warrants | $ | $ | ||||||
| Issuance of common stock on vesting of restricted stock units | $ | $ | ||||||
| In-process research and development in accrued expenses | $ | $ | ||||||
| Common stock issuance costs in accrued expenses | $ | $ | ||||||
See accompanying notes to consolidated financial statements
| -92- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
1. Organization and description of business
Description of business
Sonnet BioTherapeutics, Inc. (“Prior Sonnet”) was incorporated as a New Jersey corporation on April 6, 2015. Prior Sonnet completed a merger with publicly-held Chanticleer Holdings, Inc. (“Chanticleer”) on April 1, 2020. After the merger, Chanticleer changed its name to Sonnet BioTherapeutics Holdings, Inc. (“Sonnet” or the “Company”). Sonnet is a clinical stage, oncology-focused biotechnology company with a proprietary platform for innovating biologic medicines of single- or bi-specific action. Known as FHAB™ (Fully Human Albumin Binding), the technology utilizes a fully human single chain antibody fragment (scFv) that binds to and “hitch-hikes” on human serum albumin (“HSA”) for transport to target tissues. Sonnet’s lead proprietary asset, SON-1010, is a fully human version of Interleukin 12 (“IL-12”), covalently linked to the FHAB construct, for which Sonnet intends to pursue clinical development in solid tumor indications, including non-small cell lung cancer and head and neck cancer. Sonnet has completed a nonhuman primate (“NHP”) GLP toxicity study and has successfully manufactured drug product for clinical use. In March 2022, the FDA cleared Sonnet’s Investigational New Drug (“IND”) application for SON-1010. This allowed the Company to initiate a U.S. clinical trial (SB101) in oncology patients with solid tumors during the second calendar quarter of 2022. In September 2021, the Company created a wholly-owned Australian subsidiary, SonnetBio Pty Ltd, for the purpose of conducting certain clinical trials. Sonnet received approval and initiated an Australian clinical study (SB102) of SON-1010 in healthy volunteers during the third calendar quarter of 2022. Initial safety and tolerability data from the SB101 and SB102 studies was presented during the fourth calendar quarter of 2022, and initial pharmacokinetic and pharmacodynamic data from both studies is expected during the second calendar quarter of 2023.
The Company acquired the global development rights to its most advanced compound, SON-080, a fully human version of Interleukin 6 (“IL-6”), in April 2020 through its acquisition of the outstanding shares of Relief Therapeutics SA. Sonnet is advancing SON-080 in target indications of Chemotherapy-Induced Peripheral Neuropathy (“CIPN”) and Diabetic Peripheral Neuropathy (“DPN”). Sonnet received approval to initiate an ex-U.S. Phase 1b/2a study with SON-080 in CIPN. This study could yield initial clinical safety data during the first half of 2023. Pursuant to a license agreement the Company entered with New Life Therapeutics Pte, Ltd. (“New Life”) of Singapore in May 2021, Sonnet and New Life will be jointly responsible for developing SON-080 in DPN. The objective will be to initiate a Phase 2 study in the second half of 2023, once the CIPN safety data is available.
SON-1210 (IL12-FHAB-IL15), Sonnet’s lead bi-specific construct, combines FHAB with fully human IL-12 and fully human Interleukin 15 (“IL-15”). This compound is being developed for solid tumor indications, including colorectal cancer. A non-human primate (“NHP”) study is on track to complete in the fourth calendar quarter of 2022, and Sonnet expects to initiate the regulatory authorization process in the first half of 2023. Manufacturing and drug supply are secured with a lyophilized drug product formulation being prepared during the first calendar quarter of 2023, which is expected to be suitable for first-in-human clinical trials.
Cell line development and process development of SON-1410 (IL18-FHAB-IL12), a bi-specific combination of Interleukin 18 (“IL-18”) and IL-12, is ongoing, with early experimental drug supply suitable for formulation and analytical method development activities, in addition to small quantities for use in early development proof-of-concept in vitro studies. Process development activities will continue through 2023, with the potential to generate drug suitable for initial in vivo mouse studies by the end of the 2023 calendar year.
The Company has completed sequence confirmation for SON-3015 (anti-IL6-FHAB-anti-TGFβ). Early-stage bi-specific drug has been generated and has been stored for future use in in vivo mice studies.
| -93- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
Global pandemic - COVID-19
On March 10, 2020, the World Health Organization characterized the novel COVID-19 virus as a global pandemic. There is significant uncertainty as to the likely effects of this disease which may, among other things, materially impact the Company’s planned clinical trials. This pandemic or outbreak could result in difficulty securing clinical trial site locations, clinical research organizations (“CROs”), and/or trial monitors and other critical vendors and consultants supporting the trial. In addition, outbreaks or the perception of an outbreak near a clinical trial site location could impact the Company’s ability to enroll patients. These situations, or others associated with COVID-19, could cause delays in the Company’s clinical trial plans and could increase expected costs, all of which could have a material adverse effect on the Company’s business and its financial condition.
Liquidity
The
Company has incurred recurring losses and negative cash flows from operations since inception and it expects to generate losses from
operations for the foreseeable future primarily due to research and development costs for its potential product candidates. The
Company believes its cash of $
The
Company entered into an At-the-Market Sales Agreement with BTIG, LLC (“BTIG”) on August 15, 2022 (the “2022 Sales
Agreement”). Pursuant to the 2022 Sales Agreement, the Company may offer and sell, from time to time, through BTIG, as sales
agent and/or principal, shares of its common stock having an aggregate offering price of up to $
The Company plans to secure additional capital in the future through equity or debt financings, partnerships, collaborations, or other sources to carry out the Company’s planned development activities. If additional capital is not available when required, the Company may need to delay, curtail or cease its operations until such funding is received. Various internal and external factors will affect whether and when the Company’s product candidates become approved for marketing and successful commercialization. The regulatory approval and market acceptance of the Company’s product candidates, length of time and cost of developing and commercializing these product candidates and/or failure of them at any stage of the approval process will materially affect the Company’s financial condition and future operations.
| -94- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
Operations since inception have consisted primarily of organizing the Company, securing financing, developing its technologies through performing research and development and conducting preclinical studies. The Company faces risks associated with companies whose products are in development. These risks include the need for significant additional financing to complete its research and development, achieving its research and development objectives, defending its intellectual property rights, recruiting and retaining skilled personnel, and dependence on key members of management.
2. Summary of Significant Accounting Policies
a. Basis of presentation
The accompanying consolidated financial statements have been prepared in conformity with U.S. generally accepted accounting principles (“U.S. GAAP”). Any reference in these notes to applicable guidance is meant to refer to U.S. GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASU”) promulgated by the Financial Accounting Standards Board (“FASB”).
b. Consolidation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation.
c. Use of estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Significant estimates and assumptions reflected in these consolidated financial statements include the accrual of research and development expenses. Estimates and assumptions are periodically reviewed in-light of changes in circumstances, facts and experience. Changes in estimates are recorded in the period in which they become known. Actual results could differ from management’s estimates.
d. Segment information
Operating segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the chief operating decision maker, or decision-making group, in deciding how to allocate resources and assess performance. The Company views its operations and manages its business in one segment.
e. Fair value of financial instruments
Management believes that the carrying amounts of the Company’s financial instruments, including accounts payable, approximate fair value due to the short-term nature of those instruments.
| -95- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
f. Property and equipment
Property and equipment are recorded at cost and depreciated using the straight-line method over the estimated useful lives of the assets. Expenditures for repairs and maintenance that do not extend the estimated useful life or improve an asset are expensed as incurred. Upon retirement or sale, the cost and related accumulated depreciation and amortization of assets disposed of are removed from the accounts, and any resulting gain or loss is included in the consolidated statement of operations.
g. Impairment of long-lived assets
The Company reviews long-lived assets, such as property and equipment, for impairment whenever events or changes in circumstances indicated that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the undiscounted future cash flows expected to be generated by that asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, than an impairment charge is recognized for the amount by which the carrying value of the asset exceeds the estimated fair value of the asset. There were no impairment charges recorded during the fiscal years ended September 30, 2022 and 2021.
h. Deferred offering costs
Legal
and other costs incurred in relation to equity offerings are capitalized as deferred offering costs and charged against the proceeds
from equity offerings when received. If a financing is abandoned, deferred offering costs are expensed. As of September 30, 2022, the
Company had $
i. Collaboration revenue
Collaboration arrangements may contain multiple components, which may include (i) licenses; (ii) research and development activities; and (iii) the manufacturing and supply of certain materials. Payments pursuant to these arrangements may include non-refundable payments, upfront payments, milestone payments upon the achievement of significant regulatory and development events, sales milestones and royalties on product sales. The amount of variable consideration is constrained until it is probable that the revenue is not at a significant risk of reversal in a future period.
In determining the appropriate amount of revenue to be recognized as the Company fulfills its obligations under a collaboration arrangement, the Company performs the following steps: (i) identification of the promised goods or services in the contract; (ii) determination of whether the promised goods or services are performance obligations, including whether they are capable of being distinct; (iii) measurement of the transaction price, including the constraint on variable consideration; (iv) allocation of the transaction price to the performance obligations; and (v) recognition of revenue as the Company satisfies each performance obligation.
The Company applies significant judgment when evaluating whether contractual obligations represent distinct performance obligations, allocating transaction price to performance obligations within a contract, determining when performance obligations have been met, and assessing the recognition of variable consideration. When consideration is received prior to the Company completing its performance obligation under the terms of a contract, a contract liability is recorded as deferred income. Deferred income expected to be recognized as revenue within the twelve months following the balance sheet date is classified as a current liability. In May 2021, the Company entered into a License Agreement (the “New Life Agreement”) with New Life. See Note 6 for further discussion of the New Life Agreement.
j. Research and development expense
Research and development expenses include all direct and indirect costs associated with the development of the Company’s biopharmaceutical products. These expenses include personnel costs, consulting fees, and payments to third parties for research, development, and manufacturing services. These costs are charged to expense as incurred.
At the end of the reporting period, the Company compares payments made to third-party service providers to the estimated progress toward completion of the related project, based on the measure of progress as defined in the contract. Factors the Company considers in preparing the estimates include costs incurred by the service provider, milestones achieved, and other criteria related to the efforts of its service providers. Such estimates are subject to change as additional information becomes available. Depending on the timing of payment to the service providers and the progress that the Company estimates has been made as a result of the service provided, the Company will record a prepaid expense or accrued liability relating to these costs. Upfront milestone payments made to third parties who perform research and development services on the Company’s behalf are expensed as services are rendered. Contingent development or regulatory milestone payments are recognized upon the related resolution of such contingencies.
| -96- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
k. Foreign currency
Transaction gains and losses resulting from exchange rate changes on transactions denominated in currencies other than the U.S. dollar are included in operations in the period in which the transaction occurs and reported within the foreign exchange loss line item in the consolidated statements of operations.
The Company measures equity classified share-based awards granted to employees and nonemployees based on the estimated fair value on the date of grant and recognizes compensation expense of those awards over the requisite service period, which is the vesting period of the respective award. The Company accounts for forfeitures as they occur. For share-based awards with service-based vesting conditions, the Company recognizes compensation expense on a straight-line basis over the service period. The Company classifies share-based compensation expense in its consolidated statements of operations in the same manner in which the award recipient’s payroll costs are classified or in which the award recipient’s service payments are classified.
m. Other income
In March 2020, the U.S. federal government enacted the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”), which included a provision for a Paycheck Protection Program (“PPP”). In May 2020, the Company received a PPP loan of $0.1 million. Under the terms of the CARES Act, the Company’s PPP loan and the related interest were forgiven in full in June 2021. Forgiveness of the PPP loan is included in other income within the consolidated statement of operations for the year ended September 30, 2021.
n. Income taxes
The Company uses the asset-and-liability method of accounting for income taxes. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and operating loss and credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized. The Company recognizes the benefit of an uncertain tax position that it has taken or expects to take on its income tax return if such a position is more likely than not to be sustained.
o. Reverse stock split
On
September 16, 2022, the Company filed a Certificate of Amendment to its Certificate of Incorporation, as amended, with the Secretary
of State of the State of Delaware, which effected a
| -97- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
Basic net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding during each period (and potential shares of common stock that are exercisable for little or no consideration). Included in basic weighted-average number of shares of common stock outstanding during the years ended September 30, 2022 and 2021 are the Series B warrants with an exercise price of $ per share.
Diluted loss per share includes the effect, if any, from the potential exercise or conversion of securities such as common stock warrants and stock options which would result in the issuance of incremental shares of common stock. For diluted net loss per share, the weighted-average number of shares of common stock is the same for basic net loss per share due to the fact that when a net loss exists, dilutive securities are not included in the calculation as the impact is anti-dilutive.
| September 30, | ||||||||
| 2022 | 2021 | |||||||
| Common stock warrants | ||||||||
| Underwriter warrants | ||||||||
| Private warrants | ||||||||
| Chanticleer warrants | ||||||||
| Series C warrants | ||||||||
| Series 3 warrants | ||||||||
| Unvested restricted stock units | ||||||||
q. Recent accounting pronouncements
Recently announced
In May 2021, the FASB issued ASU 2021-04, Earnings Per Share (Topic 260), Debt-Modifications and Extinguishments (Subtopic 470-50), Compensation-Stock Compensation (Topic 718), and Derivatives and Hedging-Contracts in Entity’s Own Equity (Subtopic 815-40): Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options. The amendments in ASU 2021-04 provide guidance to clarify and reduce diversity in an issuer’s accounting for modifications or exchanges of freestanding equity-classified written call options (for example, warrants) that remain equity classified after modification or exchange. The guidance is effective for fiscal years beginning after December 15, 2021, including interim periods therein, and early adoption is permitted. The Company is currently evaluating the impact of the adoption of this pronouncement on its consolidated financial statements.
Recently adopted
In December 2019, the FASB issued ASU 2019-12, Income Taxes Topic 740 - Simplifying the Accounting for Income Taxes, which intended to simplify various aspects related to accounting for income taxes. ASU 2019-12 removes certain exceptions to the general principles in Topic 740 and also clarifies and amends existing guidance to improve consistent application of Topic 740. The adoption of ASU 2019-12 on October 1, 2021 did not have any impact on the consolidated financial statements.
In August 2020, the FASB issued ASU 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815 – 40). ASU 2020-06 eliminated the beneficial conversion and cash conversion accounting models in ASC 470-20 that required separate accounting for embedded conversion features and simplifies the settlement assessment to determine whether a contract qualifies for equity classification. In addition, the new guidance requires entities to use the if-converted method to calculate earnings per share for all convertible instruments and to include the effect of share settlement for instruments that may be settled in cash or shares. The Company adopted ASU 2020-06 on October 1, 2021 using the modified retrospective approach and applied the guidance to all financial instruments that were outstanding as of the beginning of 2021. There was no cumulative effect adjustment to the opening balance of retained earnings as a result of adopting ASU 2020-06.
| -98- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
3. Accrued Expenses
Accrued expenses consisted of the following:
| September 30, | ||||||||
| 2022 | 2021 | |||||||
| Professional fees | $ | $ | ||||||
| Compensation and benefits | ||||||||
| Research and development | ||||||||
| Other | ||||||||
| $ | $ | |||||||
4. Leases
In
December 2019, the Company entered a 36-month lease for office space in Princeton, New Jersey, which commenced February 1, 2020. In May
2022, the Company amended the existing lease agreement in order to increase the lease term by approximately three years, which has been
accounted for as a lease modification. The operating lease right-of-use asset and liability were remeasured at the modification date,
resulting in an increase to both balances of $
The components of lease expense for the years ended September 30, 2022 and 2021 are as follows:
| Lease expense | 2022 | 2021 | ||||||
| Operating lease expense | $ | $ | ||||||
| Short-term lease expense | ||||||||
| Total lease cost | $ | $ | ||||||
At
September 30, 2022, the weighted average remaining lease term was
Cash flow information related to operating leases for the years ended September 30, 2022 and 2021 is as follows:
| 2022 | 2021 | |||||||
| Cash paid for amounts included in the measurement of lease liabilities: | ||||||||
| Operating cash flows from operating leases | $ | $ | ||||||
| -99- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
Future minimum lease payments under non-cancellable leases at September 30, 2022 are as follows:
| Fiscal year | ||||
| 2023 | $ | |||
| 2024 | ||||
| 2025 | ||||
| 2026 | ||||
| Total undiscounted lease payments | ||||
| Less: imputed interest | ( | ) | ||
| Total lease liabilities | $ | |||
5. Commitments and Contingencies
Legal proceedings
From time to time, the Company is a party to various lawsuits, claims, and other legal proceedings that arise in the ordinary course of its business. While the outcomes of these matters are uncertain, management does not expect that the ultimate costs to resolve these matters will have a material adverse effect on the Company’s consolidated financial position, results of operations, or cash flows.
License agreements
In
July 2012, the Company entered into a Discovery Collaboration Agreement (the “Collaboration Agreement”) with XOMA (US) LLC
(“XOMA”), pursuant to which XOMA granted to the Company a non-exclusive, non-transferable license and/or right to use certain
materials, technologies and related information related to discovery, optimization and development of antibodies and related proteins
and to develop and commercialize products thereunder. The Company is obligated to make contingent milestone payments to XOMA totaling
$
In August 2015, the Company entered into a License Agreement (the “ARES License Agreement”) with Ares Trading, a wholly-owned subsidiary of Merck KGaA (“ARES”). Under the terms of the ARES License Agreement, ARES has granted the Company a sublicensable, exclusive, worldwide, royalty-bearing license on proprietary patents to research, develop, use and commercialize products using atexakin alfa (“Atexakin”), a low dose formulation of human IL-6 in peripheral neuropathies and vascular complications. Pursuant to the ARES License Agreement, the Company will pay ARES high single-digit royalties on net sales of products sold by the Company. Royalties are payable on a product-by-product and country-by-country basis until the later of (i) a specified period of time after the first commercial sale in such country, and (ii) the last date on which such product is covered by a valid claim in such country.
| -100- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
In
January 2019, the Company entered into a Frame Services and License Agreement (the “Cellca Agreement”) with Sartorius Stedim
Cellca GMBH (“Cellca”), pursuant to which Cellca has granted the Company a worldwide, non-exclusive, perpetual, non-transferable
license to develop, manufacture or have manufactured, use, sell, import, export and/or otherwise commercialize product based on Cellca’s
work to generate a specified transfected cell line and develop an upstream production process for such cell line. The Cellca Agreement
is effective unless terminated by either party by giving six months notice, or by giving 14 days notice if terminated for good cause.
In
October 2021, the Company entered into a Non-Exclusive License Agreement (the “Brink Agreement”) with Brink Biologics Inc.
(“Brink”), pursuant to which Brink has granted the Company a non-exclusive, non-transferable license and limited right to
sublicense certain materials and related information to develop cell-based assays for batch, quality control, stability, efficacy, potency
or any other type of assay required for production and commercialization of products. The Brink Agreement was effective November 1, 2020,
has an initial term of three years, and will automatically renew for one year terms unless terminated with written notice by either party
or if the Company ceases to use the licensed property for the product development phase. During the product development phase, the Company
is obligated to make annual license fee payments of approximately $
In
February 2022, the Company entered into a Biological Materials License Agreement (the “InvivoGen Agreement”) with InvivoGen
SAS (“InvivoGen”), pursuant to which InvivoGen has granted the Company a worldwide, non-exclusive license to use certain
reporter cells for research, development and/or quality control purposes. The InvivoGen Agreement has an initial term of three years
and may be extended for two additional three-year periods upon written notice by the Company and payment of an approximately €
In
March 2022, the Company entered into a Material Transfer and License Agreement (the “ProteoNic Agreement”) with ProteoNic
B.V. (“ProteoNic”), pursuant to which ProteoNic has granted to the Company a non-exclusive, non-transferable, non-sublicensable
(except as provided for in the ProteoNic Agreement) license for certain materials, including plasmids and DNA sequences used to generate
the vectors used in the Company’s cell lines, for the Company’s use in research, development and commercialization of product.
The license will continue until terminated by either party. The Company is obligated to make contingent milestone payments to ProteoNic
totaling up to €
| -101- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
Research and development agreements
In December 2021, the Company entered into a Research and Development Agreement (the “Navigo Agreement”) with Navigo Proteins GmbH (“Navigo”), pursuant to which Navigo will perform specified evaluation and development procedures to evaluate certain materials to determine their commercial potential. Under the terms of the Navigo Agreement, the Company granted Navigo a royalty-free, non-exclusive, worldwide, non-sublicensable, non-transferable right and license to use certain technology to perform the evaluation and development activities, and Navigo granted the Company (i) an exclusive, worldwide, perpetual, irrevocable, sublicensable, transferable, royalty-free right and license to research, develop, use, sell, have sold, distribute, import or otherwise commercially exploit certain materials, and (ii) a non-exclusive, worldwide, perpetual, sublicensable, non-transferable right and license to make or have made such materials. The Company paid a $ million technology access fee which was recorded as acquired in-process research and development and included in research and development expenses in the consolidated statement of operations for the year ended September 30, 2022. The Company is obligated to make contingent milestone payments to Navigo totaling up to $ million upon the achievement of certain evaluation and development milestones as outlined in the Navigo Agreement.
Employment agreements
The Company has entered into employment contracts with its officers and certain employees that provide for severance and continuation of benefits in the event of termination of employment either by the Company without cause or by the employee for good reason, both as defined in the contract. In addition, in the event of termination of employment following a change in control, as defined, either by the Company without cause or by the employee for good reason, any unvested portion of the employee’s initial stock option grant becomes immediately vested.
6. Collaboration Agreement
Under the New Life Agreement, the Company granted New Life an exclusive license (with the right to sublicense) to develop and commercialize pharmaceutical preparations containing a specific recombinant human IL-6, SON-080 (the “Compound”) (such preparations, the “Products”) for the prevention, treatment or palliation of diabetic peripheral neuropathy in humans (the “DPN Field”) in Malaysia, Singapore, Indonesia, Thailand, Philippines, Vietnam, Brunei, Myanmar, Lao PDR and Cambodia (the “Exclusive Territory”). New Life had the option to expand (1) the field of the exclusive license to include the prevention, treatment or palliation of chemotherapy-induced peripheral neuropathy in humans (the “CIPN Field”), which option was non-exclusive and expired on December 31, 2021; and/or (2) the territorial scope of the license to include the People’s Republic of China, Hong Kong and/or India, which option was exclusive and expired on December 31, 2021.
The Company will retain all rights to manufacture Compounds and Products anywhere in the world. The Company and New Life shall enter into a follow-on supply agreement pursuant to which the Company shall supply to New Life Products for development and commercialization thereof in the DPN Field in the Exclusive Territory on terms to be negotiated by the parties. The Company will also assist in transferring certain preclinical and clinical development know-how that is instrumental in New Life’s ability to benefit from the license.
New Life will bear the cost of, and be responsible for, among other things, conducting clinical studies and additional non-clinical studies and other developmental and regulatory activities for and commercializing Products in the DPN Field in the Exclusive Territory.
New
Life paid the Company a $
The New Life Agreement will remain in effect on a Product-by-Product, country-by-country basis and will expire upon the expiration of the Royalty Term for the last-to-expire Product in the last-to-expire country, subject to (i) each party’s early termination rights including for material breach or insolvency or bankruptcy of the other party and (ii) the Company’s Buy Back Right and New Life’s Give Back Right (as defined below).
In addition, New Life granted to the Company an exclusive option to buy back the rights (the “Buy Back Right”) granted by the Company to New Life and the Company granted New Life the right to give back the rights (the “Give Back Right”) with respect to Products in the DPN Field in one or more countries in the Exclusive Territory on terms to be agreed upon, which options will expire upon the initiation of a Phase III Trial for the applicable Product.
| -102- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
Revenue recognition
The Company first assessed the New Life Agreement under ASC 808, Collaborative Arrangements, to determine whether the New Life Agreement or units of accounts within the New Life Agreement represent a collaborative arrangement based on the risks and rewards and activities of the parties. The Company concluded that New Life represented a customer and applied relevant guidance from ASC 606, Revenue from Contracts with Customers, to evaluate the appropriate accounting under the New Life Agreement. In accordance with this guidance, the Company identified the following obligations under the arrangement: (i) license to develop, market, import, use and commercialize the Product in the Field in the Exclusive Territory (the “License”); and (ii) transfer of know-how and clinical development and regulatory activities (“R&D Activities”). The options to expand the CIPN Field and territory as well as the future supply agreement represent optional purchases, which are accounted for as separate contracts unless they convey a material right to the customer. The Company evaluated these separate contracts and did not identify any material right to be present. The Company determined that License and the R&D services are not distinct from each other and therefore combined these material promises into a single performance obligation.
The
Company determined the initial transaction price of the single performance obligation to be $
Collaboration
revenue from the single performance obligation is being recognized over the estimated performance of the R&D services. The Company
recognized $
7. Stockholders’ (Deficit) Equity
Private placement of preferred stock
In
August 2022, the Company entered into a securities purchase agreement (the “Preferred SPA”) with several accredited investors
for the issuance and sale of (i) an aggregate of shares of its Series 3 Convertible Preferred Stock, stated value $ per share,
(ii) shares of its Series 4 Convertible Preferred Stock, stated value $ per share, and (iii) Series 3 warrants to purchase up
to
| -103- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
The
shares of the Series 3 and Series 4 Convertible Preferred Stock had no voting rights other than the right to vote with common stockholders,
as a single class, on a proposed amendment to the Company’s Certificate of Incorporation, as amended, to effect a reverse split
of the outstanding shares of common stock. Each share of Series 3 Convertible Preferred Stock was entitled to vote on an as-converted
basis, and
The
private placement closed on August 15, 2022, and the stockholders voted to approve the reverse stock split on September 15, 2022. The
Series 3 and Series 4 Convertible Preferred Stock were converted to
Common stock
Through
September 30, 2022, the Company sold an aggregate of shares of common stock pursuant to the 2022 Sales Agreement with BTIG for
gross proceeds of $
Also during the year ended September 30, 2022, the Company issued shares of common stock upon the vesting of restricted stock units.
In
August 2021, the Company completed an underwritten public offering and received gross proceeds of approximately $
In
connection with the offering, the Company granted the underwriters a 30-day overallotment option to purchase up to
In
addition, warrants to purchase
In
February 2021, the Company entered into an At-the-Market Sales Agreement with BTIG (the “2021 Sales Agreement”). Pursuant
to the 2021 Sales Agreement, the Company had the ability to offer and sell, from time to time, through BTIG, as sales agent and/or principal,
shares of its common stock, having an aggregate offering price of up to $
Also during the year ended September 30, 2021, the Company issued shares of common stock upon the vesting of restricted stock units.
| -104- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
Warrant amendments and exercises
During
the year ended September 30, 2021, the Series B warrant holders exercised warrants for proceeds of $
During
the year ended September 30, 2021, the Chanticleer warrants to purchase
During the year ended September 30, 2021, the of pre-funded warrants sold in conjunction with the 2021 public offering were net share settled, resulting in the issuance of shares of common stock.
Common stock warrants
As of September 30, 2022, the following equity-classified warrants and related terms were outstanding:
Warrants Outstanding | Exercise Price | Expiration Date | ||||||||
| Common stock warrants | $ | |||||||||
| Underwriter warrants | $ | |||||||||
| Private warrants | $ | |||||||||
| Chanticleer warrants | $ - $ | |||||||||
| Series B warrants | $ | |||||||||
| Series C warrants | $ | |||||||||
| Series 3 warrants | $ | |||||||||
As
discussed above, the Series 3 warrants were issued during 2022 in connection with the private placement of preferred stock. The net proceeds
from the private placement were allocated to the Series 3 and Series 4 Convertible Preferred Stock and the Series 3 warrants based on
their relative fair values at the issuance date, resulting in an allocation of $
| Exercise price | $ | |||
| Term | ||||
| Expected volatility | % | |||
| Dividend yield | % | |||
| Risk-free interest rate | % |
In April 2020, the Company adopted the 2020 Omnibus Equity Incentive Plan (the “Plan”). The total number of shares of common stock available for issuance under the Plan as of September 30, 2022 was . The Plan increases the amount of shares issuable under the Plan by four percent of the outstanding shares of common stock at each January 1, each year. The Plan permits the granting of share-based awards, including stock options, restricted stock units and awards, stock appreciation rights and other types of awards as deemed appropriate, in each case, in accordance with the terms of the Plan. The terms of the awards are determined by the Company’s Board of Directors.
| -105- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
Restricted stock units
Any unvested RSUs will be forfeited upon termination of services. The fair value of an RSU is equal to the fair market value of the Company’s common stock on the date of grant. RSU expense is amortized straight-line over the vesting period.
| Year Ended September 30, | ||||||||
| 2022 | 2021 | |||||||
| Research and development | $ | $ | ||||||
| General and administrative | ||||||||
| $ | $ | |||||||
The following table summarizes RSU activity under the Plan:
| RSU | Weighted Average Grant Date Fair Value | |||||||
| Unvested balance at October 1, 2020 | $ | |||||||
| Granted | $ | |||||||
| Vested | ( | ) | $ | |||||
| Forfeited | ( | ) | $ | |||||
| Unvested balance at September 30, 2021 | $ | |||||||
| Granted | $ | |||||||
| Vested | ( | ) | $ | |||||
| Forfeited | ( | ) | $ | |||||
| Unvested balance at September 30, 2022 | $ | |||||||
As of September 30, 2022, total unrecognized compensation expense relating to unvested RSUs granted was $ million, which is expected to be recognized over a weighted-average period of less than .
| -106- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
9. Income Taxes
As
of September 30, 2022, the Company had $
Due to the change in ownership provisions of the Internal Revenue Code, the availability of the Company’s net operating loss carryforwards may be subject to annual limitations, against taxable income in future periods, which could substantially limit the eventual utilization of such carryforwards. The Company has not analyzed the historical or potential impact of its equity financings on beneficial ownership and therefore no determination has been made whether the net operating loss carryforwards are subject to any Internal Revenue Code Section 382 limitation. To the extent there is a limitation, there would be a reduction in the deferred tax assets with an offsetting reduction in the valuation allowance.
When uncertain tax positions exist, the Company recognizes the tax benefit of tax positions to the extent that the benefit will more likely-than-not be realized. The determination as to whether the tax benefit will more-likely-than-not be realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances. The Company recognizes interest and penalties accrued on any unrecognized tax benefits within the provision for income taxes in its consolidated statements of operations. No unrecognized tax benefits have been recorded.
| -107- |
Sonnet BioTherapeutics Holdings, Inc.
Notes to Consolidated Financial Statements
The tax effects of the temporary differences that gave rise to deferred taxes were as follows:
| September 30, | ||||||||
| 2022 | 2021 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforwards | $ | $ | ||||||
| Research and development credit carryforwards | ||||||||
| Amortization | ||||||||
| Share-based compensation | ||||||||
| Operating lease liability | ||||||||
| Accrued expenses and other | ||||||||
| Property and equipment | ||||||||
| Gross deferred tax assets | ||||||||
| Less: valuation allowance | ( | ) | ( | ) | ||||
| Deferred tax liabilities: | ||||||||
| Operating lease right-of-use asset | ( | ) | ( | ) | ||||
| Net deferred tax assets | $ | $ | ||||||
The Company recorded no income tax expense or benefit for the years ended September 30, 2022 and 2021. A reconciliation of income tax (expense) benefit at the statutory federal income tax rate and income taxes as reflected in the consolidated financial statements is as follows:
| Year Ended September 30, | ||||||||
| 2022 | 2021 | |||||||
| U.S. federal statutory rate | ( | )% | ( | )% | ||||
| State taxes, net of federal benefit | ( | ) | ( | ) | ||||
| Change in valuation allowance | ||||||||
| Research and development credit | ( | ) | ||||||
| Permanent differences | ( | ) | ||||||
| Foreign tax rate differential | ||||||||
| Other | ||||||||
| Effective income tax rate | % | % | ||||||
In
August 2022, the U.S. enacted the Inflation Reduction Act of 2022 (“IRA”). The IRA contains a number of tax-related provisions,
including a corporate alternative minimum tax of
10. Subsequent Events
The Company has evaluated subsequent events from the balance sheet date through December 15, 2022, the date at which the consolidated financial statements were available to be issued.
As
previously announced, the Company is not in compliance with Nasdaq Listing Rule 5550(b)(1) (the “Rule”), which requires companies
listed on The Nasdaq Capital Market to maintain stockholders’ equity of at least $
On
November 9, 2022, the Company filed a prospectus supplement registering the offer and sale of shares of its common stock for an aggregate
offering price of up to an additional $
Subsequent
to September 30, 2022 and through the date of this filing, the Company has sold shares of common stock for gross proceeds of
$
| -108- |
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Conclusions Regarding the Evaluation of Our Disclosure Controls
We maintain disclosure controls and procedures that are designed to provide reasonable assurance that material information required to be disclosed in our periodic reports filed under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms and to provide reasonable assurance that such information is accumulated and communicated to our management, our Chief Executive Officer and our Chief Financial Officer, to allow timely decisions regarding required disclosure. We carried out an evaluation, under the supervision and with the participation of our management, including our principal executive and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rule 13(a)-15(e) under the Exchange Act. Based on this evaluation, our principal executive officer and principal financial officer concluded that, as of September 30, 2022, our disclosure controls and procedures were effective.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Internal control over financial reporting is a process designed to provide reasonable assurance of the reliability of financial reporting and of the preparation of financial statements for external reporting purposes, in accordance with U.S. generally accepted accounting principles.
Internal control over financial reporting includes policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect transactions and disposition of assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with the authorization of its management and directors; and (3) provide reasonable assurance regarding the prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on tis financial statements.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on criteria established in the framework in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our management concluded that our internal control over financial reporting was effective as of September 30, 2022.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
This Annual Report does not include an attestation report of our independent registered public accounting firm because we are a “non-accelerated filer,” and may take advantage of certain exemptions from various reporting requirements that are applicable to public companies that are accelerated filers, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act.
Changes in Internal Controls over Financial Reporting
There was no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f)) under the Exchange Act) that occurred during the fourth quarter ended September 30, 2022, that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
| -109- |
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Directors
The following table sets forth certain information about the current directors of the Company. Directors are elected to hold office until the next annual meeting of stockholders and until their successors are elected and qualified.
| Directors | Age | Year First Became Director | ||
| Pankaj Mohan, Ph.D | 58 | 2020 | ||
| Nailesh Bhatt | 50 | 2020 | ||
| Albert Dyrness | 60 | 2020 | ||
| Donald Griffith | 74 | 2020 | ||
| Raghu Rao | 60 | 2020 | ||
| Lori McNeill | 50 | 2022 |
Set forth below are brief biographical descriptions of the individuals currently serving as the Company’s directors, based on information furnished to the Company by such individuals.
Pankaj Mohan, Ph.D.
Pankaj Mohan, Ph.D. founded Sonnet in 2015 and has since served as a member of its board of directors, and was appointed to our Board of Directors (the “Board”) as Chairman at the closing of the Merger. Dr. Mohan became the Chairman of Sonnet in June 2018 and the Chief Executive Officer of Sonnet in January 2019 and was appointed President and Chief Executive Officer of the Company at the closing of the Merger. From January 2011 to June 2018, he served as the President, Chief Executive Officer and Chairman of Oncobiologics, Inc. (now Outlook Therapeutics, Inc.; Nasdaq: OTLK), a company that he founded in 2011. Previously, Dr. Mohan served as head of Business Operations and Portfolio Management of Biologics Process and Product Development at Bristol-Myers Squibb Company and as a Director of Bioprocess Engineering at Genentech, Inc. Prior to that, Dr. Mohan served as a senior manager at Eli Lilly and Company. From May 1993 to April 1996, Dr. Mohan served as Assistant Professor (Lecturer/Fellow) at the Advanced Centre for Biochemical Engineering, University College London, London, United Kingdom. Dr. Mohan received a Ph.D. in Biochemical Engineering from the School of Chemical Engineering, University of Birmingham, Birmingham, United Kingdom, a Masters in Financial Management from Middlesex University Business School, London, United Kingdom, an Executive Management Program (AMP) from Fuqua School of Business at Duke University and a Bachelor of Chemical Engineering from the Indian Institute of Technology in Roorkee, India. He is also an author of an industry reference book on bioprocess operations (McGraw Hill). The Company believes Dr. Mohan is capable of making valuable contributions to the Board due to his extensive knowledge of the biopharmaceutical industry and his prior experience as an executive officer.
Nailesh Bhatt
Nailesh Bhatt has served on Sonnet’s board of directors since July 2018, and was appointed to our Board at the closing of the Merger. Since January 2018, Mr. Bhatt has been the Chief Executive Officer and a Board Member of VGYAAN Pharmaceuticals LLC, a company focused on developing and commercializing clinically critical drugs. Prior to that, in November 2001, Mr. Bhatt founded Proximare and is its Managing Director. Proximare is a strategic advisory firm focused exclusively on the pharmaceutical industry. Mr. Bhatt also serves as Board Member of Azurity Pharmaceuticals, Inc. since April 2018. In June 2015, Mr. Bhatt founded Proximare Lifesciences Fund. Mr. Bhatt pursued a Bachelor of Arts at Boston University with a major in Biology. The Company believes Mr. Bhatt can make valuable contributions to the Board due to his years of experience in the pharmaceutical industry working with start-ups to Fortune 500 companies.
| -110- |
Albert Dyrness
Albert Dyrness has served on Sonnet’s board of directors since October 2019, and was appointed to our Board at the closing of the Merger. Mr. Dyrness is a recognized biopharmaceutical industry expert in bio-process engineering with expertise in upstream, downstream, and fill/finish processes. Since July 2019, Mr. Dyrness has been the Managing Director of ADVENT Engineering Services, Inc., a Trinity Consultants Company, which serves as its life-sciences division. In 1988, Mr. Dyrness Co-Founded ADVENT Engineering Services, Inc., an engineering consulting firm serving the energy and life sciences industries. Starting with only 4 employees in the San Francisco Bay Area, ADVENT has grown to a staff of over 130 engineers with offices in Toronto, Canada, Singapore, Raleigh, North Carolina, Portland Oregon, Boston, Massachusetts, Irvine and San Ramon, California. In 2016, Mr. Dyrness became President and Chief Technical Officer of ADVENT and, in 2017, guided the company to a merger with Trinity Consultants, a 700-person engineering consulting firm. He also served as a member of the board of directors of Oncobiologics, Inc. (now Outlook Therapeutics, Inc.; Nasdaq: OTLK) from December 2015 to September 2017. In 1986, Mr. Dyrness graduated from the Massachusetts Institute of Technology where he studied mechanical engineering and entrepreneurism. The Company believes Mr. Dyrness is capable of making valuable contributions to the Board due to his years of experience in a Nasdaq-listed public company, along with years of entrepreneurial experience, including in the biopharmaceutical industry.
Donald Griffith, CPA
Donald Griffith, CPA has served on Sonnet’s board of directors since its inception in April 2015, was Chairman of the Sonnet board from April 2015 to June 2018, and was appointed to our Board at the closing of the Merger. Mr. Griffith has served as Sonnet’s Financial Controller since January 1, 2019, and since the Merger serves as our Controller. Prior to being Financial Controller, he served as Sonnet’s Chief Executive Officer and Chief Financial Officer from April 2015 to December 2016. Before that, Mr. Griffith was the Chief Financial Officer, Director and Secretary of Oncobiologics Inc. (now Outlook Therapeutics; Nasdaq: OTLK) from 2011 to 2018. Mr. Griffith has over 40 years’ experience in finance and accounting and is the founder and Partner of Stolz & Griffith, LLC, a New Jersey accounting firm. The Company believes Mr. Griffith is capable of making valuable contributions to the Board due to his years of experience in finance as well as in the pharmaceutical industry.
Raghu Rao
Raghu Rao has served on Sonnet’s board of directors since November 2019, and was appointed to our Board at the closing of the Merger. Mr. Rao is a serial entrepreneur, strategic business advisor and angel investor. Mr. Rao has founded, scaled and had successful exits with several high-technology companies. In his 33-year career, Mr. Rao has advised clients on the strategy and roll-out of high-profile projects, such as USA.gov, TSA Screening Gateway, Cancer.gov and other eGovernment initiatives. As the Vistage Princeton Chair, from July 2012 to March 2017, Mr. Rao ran three high-performing peer advisory boards for middle-market CEOs and business leaders of companies with total revenues exceeding $2 Billion. As the Chairman & President of InfoZen from August 1995 to July 2008, Mr. Rao has managed over $1 Billion in U.S. Federal Government contracts. Mr. Rao is a 20-year Charter Member of The Indus Entrepreneurs (TiE.org) and a 5-year patron of the Indiaspora. He has held board positions at several companies including Cellix BioSciences (Jan 2016 - Jan 2017), Paper Battery Company (Jan 2009 - Dec 2018), Kovid Group (Feb 2016 - Oct 2017) , WizNucleus (Jun 2010 - present) and InfoZen (Aug 1995 - Jul 2008). Mr. Rao is active in social entrepreneurship and community service. He co-founded the Hindu Jewish Coalition in December 2012 and Forum for Religious Freedom in March 2007, to preserve religious diversity worldwide. He has held non-profit board positions at the Infinity Foundation (New Jersey), Arsha Vidya Gurukulam (Pennsylvania) and the Family Services Agency (Maryland). Mr. Rao has an MBA in Finance from George Washington University (Dec 1991), an M.S. in Computer Science from Virginia Tech (Dec 1986), and a B.Tech. in Electrical Engineering from Indian Institute of Technology Madras (June 1984). The Company believes Mr. Rao is capable of making valuable contributions to the Board due to his 15 years of experience as an executive, along with 25 years of entrepreneurial experience, including in the biotech industry.
| -111- |
Lori McNeill
Lori McNeill has served on our Board since September 2022 and as Chairperson of our Business Advisory Committee since September 2019. Ms. McNeill is the founder and Chief Executive Officer of McNeill Consulting, LLC since 2016, a management consulting company focused on developing leaders to be more effective and ensuring that change management initiatives are seamless. Ms. McNeill has over twenty years’ experience in the healthcare industry, thirteen of which were at Pfizer Inc., which included working as the Chief of Staff of Global Operations in the Integrated Health Business unit. From 2020 to 2021, Ms. McNeill was the Chief Operating Officer and Chairperson of the board of directors of Global PPE, Inc., a worldwide supplier of personal protective equipment and safety supplies focused on healthcare and government entities to fight the COVID-19 pandemic. She has been recognized by several institutions: Top 100 Global Women in Leadership - Global Council for the Promotion of International Trade, 2021; Changemakers Summit Award Winner, 2021; The State of Women in Leadership – Cover article for HR.com, 2020; and Pfizer International Innovation Excellence Award, 2011 and is currently Global Chairperson of Womenomics. The Company believes Ms. McNeill is capable of making valuable contributions to the Board due to her over 20 years of experience in the healthcare industry, including in senior leadership positions.
Executive Officers
The following table sets forth certain information about the current executive officers of the Company:
| Executive Officers | Age | Position and Office | ||
| Pankaj Mohan, Ph.D | 58 | President and Chief Executive Officer | ||
| Jay Cross | 52 | Chief Financial Officer | ||
| John K. Cini, Ph.D. | 70 | Chief Scientific Officer | ||
| Susan Dexter | 67 | Chief Technical Officer | ||
| Richard Kenney, M.D. | 64 | Chief Medical Officer |
Set forth below are brief biographical descriptions of the individuals currently serving as the Company’s executive officers, based on information furnished to the Company by such individuals.
Pankaj Mohan, Ph.D.
See description under Directors.
Jay Cross
Jay Cross joined Sonnet in May 2019 and has since served as its Chief Financial Officer and Chief Business Officer, and was appointed Chief Financial Officer of the Company at the closing of the Merger. Prior to Sonnet, Mr. Cross was a Managing Director with Chardan Capital’s healthcare investment banking team from November 2015 to March 2019, where he focused on biopharmaceuticals. Prior to that, from May 2014 to June 2015, Mr. Cross served as a Director with Alere Financial Partners and from May 2011 to October 2013 as a Senior Analyst at Balyasny Asset Management. He launched his career in finance in 1999 as an associate analyst covering biotechnology on the healthcare equity research team at Hambrecht & Quist. Mr. Cross earned an M.P.H. from the Yale University School of Medicine and a B.S. in psychology from Washington & Lee University.
John K. Cini, Ph.D.
John K. Cini, Ph.D. co-founded Sonnet in 2015 and has since served as its Chief Scientific Officer, and was appointed Chief Scientific Officer of the Company at the closing of the Merger, where he oversees and directs the Company’s discovery and development programs. His role includes the oversight of the selection process of cancer and immune oncology targets and proof-of-concept testing. Prior to joining Sonnet, he was Vice President of Discovery and Development Sciences at Oncobiologics, Inc. from January 2011 to April 2015. Dr. Cini has successfully advanced more than 20 novel monoclonal antibody products from discovery to IND. He is the holder of several novel product and formulation patents and applications. He has also been directly involved in several successful novel biologics through early discovery research into development and manufacturing through clinical trials and commercialization. Previous positions include Executive Director at Mederex (acquired by Bristol-Myers Squibb in 2010), lead discovery scientific roles at Johnson & Johnson (Ethicon, OrthoBioTech & Pharmaceutical Research), and Bayer. Dr. Cini’s therapeutic areas of expertise in system biology include oncology, immune oncology, inflammation, osteoporosis, wound healing, surgical adhesion and cellular aging. Dr. Cini has a PhD in Biochemistry from University of North Texas.
| -112- |
Susan Dexter
Susan Dexter has served as a contract consultant to Sonnet in the capacity of Chief Technical Officer since May 2019 and was appointed full-time Chief Technical Officer of the Company at the closing of the Merger. She came to Sonnet with more than thirty years of experience in biotechnology science, manufacturing and business development having been directly involved in three start-up companies, and multiple M&A activities. Her expertise in CMC for biologics process development ranges from cell line development to process development through commercial manufacturing. In her role as Managing Director at Latham Biopharm Group from September 2008 until the closing of the Merger, Ms. Dexter ran the Product Development service offering, managing the activities and disciplines related to pre-animal toxicology, pre-clinical tox study and CMC-related activities, including IND filings, Quality oversight of cGMP activities and other related CMC supply chain activities. She came to LBG from Xcellerex, Inc., a CDMO and developer of single use technology for bioprocessing. She was Chief Business Officer at Xcellerex from April 2004 to September 2008. Prior to Xcellerex, from July 1998 to April 2004, she was VP of Business Development at The Dow Chemical Company’s CDMO, an acquisition of Collaborative BioAlliance, facilitated by Ms. Dexter in 2000; and Assoc. Director of Business Development, at Celltech Biologics, purchased by Lonza Biologics, a biologics CDMO. She worked at Celltech/Lonza from 1986 to July 1998. Ms. Dexter holds a double major with Honors in Immunology and Marketing from American University, Washington, D.C., and certifications from Harvard University in ‘Negotiations for Lawyers’ and ‘Finance for Non-financial Managers.’ She was also Professor Emeritus at University College, London, Department of Bioengineering, teaching a credited course lecture and workshop in “Project managing biologics facility,” to graduate, Ph.D. and post-graduate professionals, from 1999 to 2006.
Richard Kenney, M.D.
Richard Kenney, M.D. has served as the Company’s Chief Medical Officer since April 2021. Dr. Kenney has more than 20 years of experience in translational-stage development of biologics, as well as the commercialization strategy and corporate management of preclinical, clinical-stage and commercialized vaccines and immunotherapies. As President of ClinReg Biologics, he has provided strategic consulting in clinical and regulatory affairs of biologics and medical monitoring and pharmacovigilance in several capacities. Dr. Kenney most recently served as Chief Development Officer at X-VAX Technology and previously held Chief Medical Officer roles at Immune Design and Crucell Holland, where he led the clinical development and regulatory affairs groups. Dr. Kenney was a researcher/reviewer for the FDA for over six years and did post-graduate training at Duke and NIH. Dr. Kenney received a B.S. in Chemistry from George Washington University and his M.D. from Harvard Medical School.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires the Company’s directors and executive, officers, and persons who are beneficial owners of more than 10% of a registered class of the Company’s equity securities, to file reports of ownership and changes in ownership with the SEC. These persons are required by SEC regulations to furnish the Company with copies of all Section 16(a) forms they file.
Based solely upon the Company’s review of copies of Forms 3, 4 and 5 furnished to the Company, the Company believes that all of its directors, executive officers and any other applicable stockholders timely filed all reports required by Section 16(a) of the Exchange Act during the fiscal year ended September 30, 2022, except for the following: Form 4s for Don Griffith, Nailesh Bhatt, Susan Dexter, Pankaj Mohan, John Cini, Raghu Rao, Jay Cross and Albert Dyrness which were due on December 17, 2022 were filed on April 8, 2022.
Code of Business Conduct and Ethics
The Company has adopted a Code of Business Conduct and Ethics that applies to its directors, officers and employees. The purpose of the Code of Business Conduct and Ethics is to deter wrongdoing and to provide guidance to the Company’s directors, officers and employees to help them recognize and deal with ethical issues, to provide mechanisms to report unethical or illegal conduct and to contribute positively to the Company’s culture of honesty and accountability. The Company’s Code of Business Conduct and Ethics is publicly available on the Company’s website at https://www.sonnetbio.com/. If the Company makes any substantive amendments to the Code of Business Conduct and Ethics or grants any waiver, including any implicit waiver from a provision of the Code of Business Conduct and Ethics to its directors or executive officers, the Company will disclose the nature of such amendments or waiver on its website or in a current report on Form 8-K.
| -113- |
Audit Committee
The Board has established an Audit Committee currently consisting of Messrs. Bhatt (Chairman), Dyrness and Rao. The Audit Committee’s primary functions are to oversee and review: the integrity of the Company’s consolidated financial statements and other financial information furnished by the Company, the Company’s compliance with legal and regulatory requirements, the Company’s systems of internal accounting and financial controls, the independent auditor’s engagement, qualifications, performance, compensation and independence, related party transactions, and compliance with the Company’s Code of Business Conduct and Ethics.
Each member of the Audit Committee is “independent” as that term is defined under the applicable rules of the SEC and the applicable rules of The Nasdaq Stock Market. The Board has determined that each Audit Committee member has sufficient knowledge in financial and auditing matters to serve on the Committee. The Board determined that Mr. Rao is an “audit committee financial expert,” as defined under the applicable rules of the SEC and the applicable rules of The Nasdaq Stock Market. The Company’s Board has adopted an Audit Committee Charter, which is available for viewing at https://www.sonnetbio.com/.
Item 11. Executive Compensation.
Summary Compensation Table
The following table shows the compensation awarded to or earned by each person serving as the Company’s principal executive officer during fiscal year 2022, the Company’s two most highly compensated executive officers who were serving as executive officers as of September 30, 2022 and up to two additional individuals for whom disclosure would have been provided but for the fact that such individuals were not serving as an executive officer as of September 30, 2022. The persons listed in the following table are referred to herein as the “named executive officers.”
SUMMARY COMPENSATION TABLE
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock awards ($)(1) | Option awards ($)(1) | All other compensation ($) | Total ($) | |||||||||||||||||||||
| Pankaj Mohan, Ph.D | 2022 | 559,729 | 218,680 | 144,061 | - | 82,923 | 1,005,393 | |||||||||||||||||||||
| President and Chief Executive Officer | 2021 | 512,236 | 279,930 | - | - | - | 792,165 | |||||||||||||||||||||
| John Cini, Ph.D. | 2022 | 413,048 | 113,899 | 36,015 | - | 52,014 | 614,976 | |||||||||||||||||||||
| Chief Scientific Officer | 2021 | 382,594 | 142,505 | - | - | - | 525,099 | |||||||||||||||||||||
| Jay Cross | 2022 | 403,676 | 127,649 | 30,128 | - | 43,358 | 604,811 | |||||||||||||||||||||
| Chief Financial Officer | 2021 | 375,767 | 156,878 | - | - | - | 532,645 | |||||||||||||||||||||
| (1) | Represents the aggregate grant date fair value for grants made in 2022 and 2021 computed in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 718. This calculation does not give effect to any estimate of forfeitures related to service-based vesting, but assumes that the executive will perform the requisite service for the award to vest in full. |
| -114- |
Narrative Disclosure to Summary Compensation Table
Employment Agreements
The material terms of each named executive officer’s employment agreement or arrangement are described below.
Sonnet entered into an employment agreement with Dr. Mohan on December 31, 2018, as amended (the “Mohan Agreement”), setting forth the terms of his employment as Chief Executive Officer, which agreement was assumed by the Company at the closing of the Merger. Pursuant to the employment agreement, Dr. Mohan is entitled to, among other things, (i) an annual gross base salary of $490,000, (ii) eligibility for a bonus equal to 5.4% of gross revenue received by the Company from a strategic transaction and (iii) for any year in which the bonus in the previous clause amounts to less than 50% of the base salary, an additional performance-based cash bonus to bring the aggregate cash bonus for such year to up to 50% of the base salary, as determined by the board. The employment agreement shall terminate in accordance with its terms. Pursuant to Dr. Mohan’s employment agreement, if he is terminated without “Cause” or for “Good Reason” within 2 months prior to or within 12 months following a “Change in Control”, he is entitled to (i) his base salary for 18 months, (ii) a bonus equal to his performance bonus for the year in which the termination occurs, divided by 12, and then multiplied by 18, and (iii) if he timely continued coverage under COBRA, payment for COBRA premiums necessary to continue coverage until the earliest of (a) 18 months following the termination date, (b) the date he becomes eligible for substantially equivalent health insurance coverage in connection with new employment or self-employment, or (c) the date he becomes ineligible for COBRA continuation coverage. If Dr. Mohan is terminated without “Cause” or for “Good Reason” not coincident with a “Change in Control”, he is entitled to (i) his base salary for 18 months, (ii) any performance bonus for the performance year in which his termination occurs, and (iii) if he timely continued coverage under COBRA, payment for COBRA premiums necessary to continue coverage until the earliest of (a) 18 months following the termination date, (b) the date he becomes eligible for substantially equivalent health insurance coverage in connection with new employment or self-employment, or (c) the date he becomes ineligible for COBRA continuation coverage.
Sonnet entered into an employment agreement with Dr. Cini on January 10, 2020, as amended (the “Cini Agreement”), setting forth the terms of his employment as Chief Scientific Officer, which agreement was assumed by the Company at the closing of the Merger. Pursuant to the employment agreement, Dr. Cini is entitled to, among other things, (i) an annual gross base salary of $370,000, (ii) eligibility for a bonus equal to 1.1% of gross revenue received by the Company from a strategic transaction and (iii) for any year in which the bonus in the previous clause amounts to less than 35% of the base salary, an additional performance-based cash bonus to bring the aggregate cash bonus for such year to up to 35% of the base salary, as determined by the board. The employment agreement shall terminate in accordance with its terms. Pursuant to Dr. Cini’s employment agreement, if he is terminated without “Cause” or for “Good Reason” within 2 months prior to or within 12 months following a “Change in Control”, he is entitled to (i) his base salary for 12 months and (ii) if he timely continued coverage under COBRA, payment for COBRA premiums necessary to continue coverage until the earliest of (a) 18 months following the termination date, (b) the date he becomes eligible for substantially equivalent health insurance coverage in connection with new employment or self-employment, or (c) the date he becomes ineligible for COBRA continuation coverage. If Dr. Cini is terminated without “Cause” or for “Good Reason” not coincident with a “Change in Control”, he is entitled to (i) his base salary for 9 months and (ii) if he timely continued coverage under COBRA, payment for COBRA premiums necessary to continue coverage until the earliest of (a) 12 months following the termination date, (b) the date he becomes eligible for substantially equivalent health insurance coverage in connection with new employment or self-employment, or (c) the date he becomes ineligible for COBRA continuation coverage.
Sonnet entered into an employment agreement with Mr. Cross on January 10, 2020 (the “Cross Agreement”), setting forth the terms of his employment as Chief Financial Officer, which agreement was assumed by the Company at the closing of the Merger. Pursuant to the employment agreement, Mr. Cross is entitled to, among other things, (i) an annual gross base salary of $365,000 and (ii) eligibility for a performance-based cash bonus of up to 40% of the base salary, as determined by the board. The employment agreement shall terminate in accordance with its terms. Pursuant to Mr. Cross’s employment agreement, if he is terminated without “Cause” or for “Good Reason” within 2 months prior to or within 12 months following a “Change in Control”, he is entitled to (i) his base salary for 12 months, (ii) any performance bonus for the performance year in which his termination occurs, and (iii) if he timely continued coverage under COBRA, payment for COBRA premiums necessary to continue coverage until the earliest of (a) 18 months following the termination date, (b) the date he becomes eligible for substantially equivalent health insurance coverage in connection with new employment or self-employment, or (c) the date he becomes ineligible for COBRA continuation coverage. If Mr. Cross is terminated without “Cause” or for “Good Reason” not coincident with a “Change in Control”, he is entitled to (i) his base salary for 9 months, (ii) any performance bonus for the performance year in which his termination occurs, and (iii) if he timely continued coverage under COBRA, payment for COBRA premiums necessary to continue coverage until the earliest of (a) 12 months following the termination date, (b) the date he becomes eligible for substantially equivalent health insurance coverage in connection with new employment or self-employment, or (c) the date he becomes ineligible for COBRA continuation coverage.
| -115- |
Other Agreements
On April 1, 2020, the Company entered into an employment agreement with Ms. Dexter (the “Dexter Agreement”), setting forth the terms of her employment as Chief Technical Officer. Pursuant to the employment agreement, Ms. Dexter is entitled to, among other things, (i) an annual gross base salary of $310,000 and (ii) eligibility for a performance-based cash bonus of up to 35% of the base salary, as determined by the board. The employment agreement shall terminate in accordance with its terms. Pursuant to Ms. Dexter’s employment agreement, if she is terminated without “Cause” or for “Good Reason” within 2 months prior to or within 12 months following a “Change in Control”, she is entitled to (i) her base salary for 12 months, (ii) any performance bonus for the performance year in which her termination occurs, and (iii) if she timely continued coverage under COBRA, payment for COBRA premiums necessary to continue coverage until the earliest of (a) 18 months following the termination date, (b) the date she becomes eligible for substantially equivalent health insurance coverage in connection with new employment or self-employment, or (c) the date she becomes ineligible for COBRA continuation coverage. If Ms. Dexter is terminated without “Cause” or for “Good Reason” not coincident with a “Change in Control”, she is entitled to (i) his base salary for 9 months, (ii) any performance bonus for the performance year in which her termination occurs, and (iii) if she timely continued coverage under COBRA, payment for COBRA premiums necessary to continue coverage until the earliest of (a) 12 months following the termination date, (b) the date she becomes eligible for substantially equivalent health insurance coverage in connection with new employment or self-employment, or (c) the date she becomes ineligible for COBRA continuation coverage.
Outstanding Equity Awards at Fiscal Year End
The following table sets forth certain information, on an award-by-award basis, concerning unexercised options to purchase common stock, restricted shares of common stock and common stock that has not yet vested for each named executive officer and outstanding as of September 30, 2022.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR END - 2022
| Stock Awards | ||||||||
| Name | Equity incentive plan awards: Number of unearned shares, units or other rights that have not vested (#) | Equity incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested ($) | ||||||
| Pankaj Mohan Ph.D. | 22,866 | (1) | 33,613 | |||||
| John Cini, Ph.D. | 5,716 | (1) | 8,403 | |||||
| Jay Cross | 4,782 | (1) | 7,030 | |||||
| (1) | Each restricted stock unit ( “RSU”) award vests 100% on January 1, 2023. |
Director Compensation
Non-Employee Director Compensation Policy
In connection with the Merger, the Board approved a new director compensation policy for its non-employee directors. Other than reimbursement for reasonable expenses incurred in connection with attending board and committee meetings, this policy provides for the following cash compensation:
● each non-employee director is entitled to receive an annual fee from us of $35,000;
● the chair of our audit committee will receive an annual fee from us of $15,000;
| -116- |
● the chair of our compensation committee will receive an annual fee from us of $10,000;
● the chair of our nominating and corporate governance committee will receive an annual fee from us of $8,000; and
● each non-chairperson member of the audit committee, the compensation committee and the nominating and corporate governance committee will receive annual fees from us of $7,500, $5,000 and $4,000, respectively.
Each non-employee director that joins the Board receives an initial option grant to purchase 0.080% of the Company’s fully-diluted outstanding Common Stock at the closing of the Merger, which shall vest 33% per year over three years, the first vesting date to occur on the one-year anniversary of the grant date. Each non-employee director also receives an annual option grant to purchase 0.040% of the Company’s fully-diluted outstanding Common Stock at the closing of the Merger, which shall vest 100% upon the earlier of the one-year anniversary of the grant date or the next annual stockholder meeting. Upon a change in control, as defined in the Company’s equity incentive plan, 100% of the shares underlying these options shall become vested and exercisable immediately prior to such change in control.
Except as set forth in the table below, the non-employee directors did not receive any cash or equity compensation during fiscal year 2022:
DIRECTOR COMPENSATION
| Name | Fees Earned or Paid in Cash ($) | Stock Awards ($)(1) | Option Awards ($)(1) | All Other Compensation ($) | Total ($) | |||||||||||||||
| Nailesh Bhatt(2) | 54,000 | 6,368 | - | - | 60,368 | |||||||||||||||
| Albert Dyrness(3) | 55,500 | 6,368 | - | - | 61,868 | |||||||||||||||
| Donald Griffith (4) | - | 6,750 | - | 134,634 | 141,384 | |||||||||||||||
| Raghu Rao(5) | 56,500 | 6,368 | - | - | 62,868 | |||||||||||||||
| Lori McNeill(6) | - | - | - | - | - | |||||||||||||||
| (1) | Represents the aggregate grant date fair value for grants made in 2022 computed in accordance with FASB ASC Topic 718. This calculation does not give effect to any estimate of forfeitures related to service-based vesting, but assumes that the executive will perform the requisite service for the award to vest in full. | |
| (2) | Mr. Bhatt holds an aggregate of 1,010 restricted stock units, as of September 30, 2022. | |
| (3) | Mr. Dyrness holds an aggregate of 1,010 restricted stock units, as of September 30, 2022. | |
| (4) | Mr. Griffith has served as Sonnet’s Financial Controller since January 1, 2019, and since the Merger serves as our Controller. The amounts in the table above under “All Other Compensation” represent salary and bonus earned by Mr. Griffith for the fiscal year 2022. See the description of the employment agreement with Mr. Griffith below. | |
| (5) | Mr. Rao holds an aggregate of 1,010 restricted stock units, as of September 30, 2022. | |
| (6) | Ms. McNeill joined the board on September 25, 2022. |
Other Agreement with a Director
Sonnet entered into an employment agreement with Mr. Griffith on January 1, 2019, setting forth the terms of his employment as Financial Controller. Pursuant to the employment agreement, Mr. Griffith is entitled to, among other things, (i) an annual prorated gross base salary of $150,000 and (ii) eligibility for a target bonus equal 25% of gross salary earned. The employment agreement has no specific term and constitutes an at-will employment.
| -117- |
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information as of December 14, 2022 with respect to the beneficial ownership of common stock of the Company by the following: (i) each of the Company’s current directors; (ii) each of the named executive officers; (iii) all of the current executive officers and directors as a group; and (iv) each person known by the Company to own beneficially more than five percent (5%) of the outstanding shares of the Company’s common stock.
For purposes of the following table, beneficial ownership is determined in accordance with the applicable SEC rules and the information is not necessarily indicative of beneficial ownership for any other purpose. Except as otherwise noted in the footnotes to the table, the Company believes that each person or entity named in the table has sole voting and investment power with respect to all shares of the Company’s common stock shown as beneficially owned by that person or entity (or shares such power with his or her spouse). Under the SEC’s rules, shares of the Company’s common stock issuable under options that are exercisable on or within 60 days after December 14, 2022 (“Presently Exercisable Options”) are deemed outstanding and therefore included in the number of shares reported as beneficially owned by a person or entity named in the table and are used to compute the percentage of the common stock beneficially owned by that person or entity. These shares are not, however, deemed outstanding for computing the percentage of the common stock beneficially owned by any other person or entity.
The percentage of the common stock beneficially owned by each person or entity named in the following table is based on 7,934,156 shares of common stock issued and outstanding as of December 14, 2022 plus any shares issuable upon exercise of Presently Exercisable Options held by such person or entity.
Title of Class | Name And Address of Beneficial Owner* | Amount And Nature of Beneficial Ownership | Percent Of Class | |||||||
| Named Executive Officers and Directors: | ||||||||||
| Pankaj Mohan, Ph.D. | 115,524 | (1) | 1.5 | % | ||||||
| Nailesh Bhatt | 1,962 | (2) | ** | |||||||
| Albert Dyrness | 2,027 | (3) | ** | |||||||
| Donald Griffith | 7,584 | (4) | ** | |||||||
| Raghu Rao | 1,604 | (5) | ** | |||||||
| Lori McNeill | - | - | ||||||||
| John. K. Cini, Ph.D. | 21,720 | (6) | ** | |||||||
| Jay Cross | 10,368 | (7) | ** | |||||||
| All current executive officers and directors as a group (10 persons) | 172,507 | 2.2 | % | |||||||
| 5% Holders | ||||||||||
| None | ||||||||||
| (*) | Unless otherwise indicated, the address is c/o Sonnet BioTherapeutics, Inc., 100 Overlook Center, Suite 102, Princeton, New Jersey, 08540. | |
| (**) | Less than 1%. | |
| (1) | Includes (i) 66,478 shares of common stock held by the Mohan Family Office, over which Dr. Mohan has shared power to vote and dispose with Swati Mohan, his spouse; (ii) 570 shares of common stock held individually by Pankhuri Mohan, Dr. Mohan’s child, over which Dr. Mohan has shared power to vote and dispose with Pankhuri Mohan; and (iii) 304 shares of common stock issuable upon exercise of warrants held by the Mohan Family Office, over which Dr. Mohan has shared power to vote and dispose with Swati Mohan, which are exercisable within 60 days of December 14, 2022. Includes 22,866 restricted stock units, which will be settled in shares of common stock and vest within 60 days of December 14, 2022. |
| -118- |
| (2) | Includes 1,010 restricted stock units, which will be settled in shares of common stock and vest within 60 days of December 14, 2022. | |
| (3) | Includes 91 shares of common stock issuable upon exercise of warrants which are exercisable within 60 days of December 14, 2022. Includes 1,010 restricted stock units, which will be settled in shares of common stock and vest within 60 days of December 14, 2022. | |
| (4) | Includes 1,071 restricted stock units, which will be settled in shares of common stock and vest within 60 days of December 14, 2022. | |
| (5) | Includes 7 shares of common stock issuable upon exercise of warrants which are exercisable within 60 days of December 14, 2022. Includes 1,010 restricted stock units, which will be settled in shares of common stock and vest within 60 days of December 14, 2022. | |
| (6) | Includes 5,716 restricted stock units, which will be settled in shares of common stock and vest within 60 days of December 14, 2022. | |
| (7) | Excludes 4,782 restricted stock units, which will be settled in shares of common stock and vest within 60 days of December 14, 2022. |
Equity Compensation Plan Information
The following table provides information as of September 30, 2022 regarding shares of the Company’s common stock that may be issued under the Company’s existing equity compensation plans, including its 2020 Omnibus Equity Incentive Plan (the “2020 Plan”).
| Equity Compensation Plan Information | ||||||||||||
| Plan Category | (a) Number of securities to be issued upon exercise of outstanding options, warrants and rights | (b) Weighted average exercise price of outstanding options, warrants and rights | (c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||||
| Equity compensation plans approved by security holders (1) | 47,798 | N/A | - | |||||||||
| Equity compensation plans not approved by security holders | N/A | N/A | N/A | |||||||||
| Total | 47,798 | - | - | |||||||||
| (1) | The weighted-average exercise price does not reflect the shares that will be issued in connection with the settlement of RSUs, since RSUs have no exercise price. Other than RSUs, there were no outstanding options, warrants, or rights under our equity compensation plan as of September 30, 2022. |
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Other than compensation arrangements for named executive officers and directors, the Company describes below each transaction and series of similar transactions, since the beginning of fiscal year 2021, to which the Company was a party or will be a party, in which:
| ● | the amounts involved exceeded or will exceed the lesser of $120,000 or one percent of the average of the smaller reporting company’s total assets at year-end for the last two completed fiscal years; and |
| -119- |
| ● | any of the Company’s directors, nominees for director, executive officers or holders of more than 5% of the Company’s common stock, or any member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest. |
Compensation arrangements for the Company’s named executive officers and directors are described in the section entitled “Executive Compensation”.
Indemnification Agreements
The Company has entered into indemnification agreements with each of its current directors and executive officers. These agreements will require the Company to indemnify these individuals to the fullest extent permitted under Delaware law against liabilities that may arise by reason of their service to the Company, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified. The Company also intends to enter into indemnification agreements with its future directors and executive officers.
Director Independence
The Company is currently managed by a six-member board of directors. The Company has determined that Messrs. Bhatt, Dyrness and Rao and Ms. McNeill are “independent” as that term is defined under the rules of The NASDAQ Stock Market.
Item 14. Principal Accountant Fees and Services.
The following table summarizes the fees for professional services rendered by KPMG LLP, the Company’s (and Sonnet’s, prior to the Merger) independent registered public accounting firms, for each of the respective last two fiscal years:
| Fee Category | 2022 | 2021 | ||||||
| Audit Fees | $ | 435,000 | $ | 509,500 | ||||
| Audit-Related Fees | - | - | ||||||
| Tax Fees | 33,500 | 34,000 | ||||||
| All Other Fees | - | - | ||||||
| Total Fees | $ | 468,500 | $ | 543,500 | ||||
Audit Fees
Represents fees for professional services provided in connection with the audit of the Company’s annual consolidated financial statements and reviews of the Company’s quarterly interim consolidated financial statements, as well as for fees associated with registration statements, comfort letters and consents.
Audit-Related Fees
We did not incur any audit-related fees from our independent auditors in 2022 or 2021.
Tax Fees
Tax fees are associated with tax compliance, tax advice, tax planning and tax preparation services.
All Other Fees
Fees related to products and services provided by the principal accountant, other than the services reported in the above sections.
| -120- |
The Audit Committee is responsible for appointing, setting compensation and overseeing the work of the independent auditors. The Audit Committee is required to review and approve the proposed retention of independent auditors to perform any proposed auditing and non-auditing services as outlined in its charter. The Audit Committee has not established policies and procedures separate from its charter concerning the pre-approval of auditing and non-auditing related services. As required by Section 10A of the Exchange Act, our Audit Committee has authorized all auditing and non-auditing services provided by KPMG LLP during 2022 and 2021 and the fees paid for such services. However, the pre-approval requirement may be waived with respect to the provision of non-audit services for the Company if the “de minimis” provisions of Section 10A(i)(1)(B) of the Exchange Act are satisfied.
The Audit Committee has considered whether the provision of Audit-Related Fees, Tax Fees, and all other fees as described above is compatible with maintaining KPMG LLP’s independence and has determined that such services for fiscal years 2022 and 2021 were compatible. All such services were approved by the Audit Committee pursuant to Rule 2-01 of Regulation S-X under the Exchange Act to the extent that rule was applicable.
The Audit Committee is responsible for reviewing and discussing the audited consolidated financial statements with management, discussing with the independent registered public accountants the matters required by Public Company Accounting Oversight Board Auditing Standard No. 1301 Communications with Audit Committees, receiving written disclosures from the independent registered public accountants required by the applicable requirements of the Public Company Accounting Oversight Board regarding the independent registered public accountants’ communications with the Audit Committee concerning independence and discussing with the independent registered public accountants their independence, and recommending to the Board that the audited consolidated financial statements be included in the Company’s Annual Report on Form 10-K.
PART IV
Item 15. Exhibits, Financial Statement Schedules.
(a)(1) Financial Statements. The financial statements filed as part of this report are listed on the Index to the Consolidated Financial Statements.
(a)(2) Financial Statement Schedules. Schedules are omitted because they are not applicable or the required information is shown in the consolidated financial statements or notes thereto.
(a)(3) Exhibits. Reference is made to the Exhibit Index. The exhibits are included, or incorporated by reference, in this annual report on Form 10-K and are numbered in accordance with Item 601 of Regulation S-K.
Item 16. Form 10-K Summary.
None.
| -121- |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Sonnet BioTherapeutics, Inc. (Registrant) | ||
| Date: December 15, 2022 | /s/ Pankaj Mohan, Ph.D | |
| Pankaj Mohan, Ph.D | ||
President and Chief Executive Officer (Principal Executive Officer and duly authorized signatory) | ||
| Date: December 15, 2022 | /s/ Jay Cross | |
| Jay Cross | ||
Chief Financial Officer (Principal Financial and Accounting Officer) |
SIGNATURES AND POWER OF ATTORNEY
KNOW ALL BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Pankaj Mohan, Ph.D and Jay Cross, and each of them, his true and lawful agent, proxy and attorney-in-fact, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to (i) act on, sign and file with the Securities and Exchange Commission any and all amendments to this annual report on Form 10-K together with all schedules and exhibits thereto, (ii) act on, sign and file such certificates, instruments, agreements and other documents as may be necessary or appropriate in connection therewith and, (iii) take any and all actions which may be necessary or appropriate to be done, as fully for all intents and purposes as he might or could do in person, hereby approving, ratifying and confirming all that such agent, proxy and attorney-in-fact or any of his substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Pankaj Mohan, Ph.D | President, Chief Executive Officer and Chairman | December 15, 2022 | ||
| Pankaj Mohan, Ph.D | (Principal Executive Officer) | |||
| /s/ Jay Cross | Chief Financial Officer | December 15, 2022 | ||
| Jay Cross | (Principal Financial and Accounting Officer) | |||
| /s/ Albert Dyrness | Director | December 15, 2022 | ||
| Albert Dyrness | ||||
| /s/ Nailesh Bhatt | Director | December 15, 2022 | ||
| Nailesh Bhatt | ||||
| /s/ Raghu Rao | Director | December 15, 2022 | ||
| Raghu Rao | ||||
| /s/ Donald Griffith | Director | December 15, 2022 | ||
| Donald Griffith | ||||
| /s/ Lori McNeill | Director | December 15, 2022 | ||
| Lori McNeill | ||||
| -122- |
INDEX TO EXHIBITS
| -123- |
| -124- |
| -125- |
| 32.1 | Certification of Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.** | |
| 32.2 | Certification of Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.** | |
| 101.INS | Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document.* | |
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document.* | |
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document.* | |
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document.* | |
| 101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document.* | |
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document.* | |
| 104 | Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101).* |
| * | Filed herewith. |
| ** | Furnished herewith. |
| *** | Filed herewith; portions of the exhibit have been omitted pursuant to Item 601(b)(10) of Regulation S-K. A copy of any omitted portions will be furnished to the Securities and Exchange Commission upon request. |
| # | The schedules and exhibits to this agreement have been omitted pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the Securities and Exchange Commission upon request. |
| † | Indicates a management contract or compensation plan, contract or arrangement. |
| -126- |